Meyers and Silliman (this volume) compare organic carbon concentrations measured by different shipboard and shorebased procedures. The shipboard procedure, which uses the difference between whole sediment total carbon measurements and carbonate carbon measurements to arrive at total organic carbon (TOC) concentrations, is slightly less reliable in sediments containing little organic carbon than is the shorebased procedure, which directly measures organic carbon concentrations of Carbonate-free samples. Our discussions of TOC concentrations and organic matter C/N ratios will consequently use the shorebased data only.
Averaged TOC concentrations of the shorebased Unit I samples are: Site 897, 0.78%; Site 898, 0.68%; Site 899, 0.44%; Site 900, 0.36%. These values are slightly higher to more than twice the average organic carbon content of 0.3% of deep-sea sediments and rocks from DSDP Legs 1 through 33 compiled by McIver (1975). Many of the samples, however, are poor in organic carbon, and their low concentrations are not related to their lithology as represented by CaCO3 concentrations (Fig. 3, Fig. 4, Fig. 5, Fig. 6). Of particular note, the uppermost lithologic units drilled at Sites 897, 898, and 899 seem to be parts of a single, continuous turbidite layer (Fig. 2; Table 1), yet the TOC concentrations at Sites 897 and 898 are significantly higher than those at Site 899, which is located between the other two. In addition, TOC concentrations vary with depth in Unit I as it is expressed at each of the four drill sites. The delivery of organic matter to the landward edge of the Iberia Abyssal Plain evidently has varied both spatially and temporally during the Pliocene and Pleistocene. Part of this variability may result from the turbidites originating from different locations on the Iberia Margin, but the primary cause is probably the greater thicknesses of the turbidites at Sites 897 and 898 relative to Sites 899 and 900, which affects the degree of postdepositional preservation of organic matter (Shaw and Meyers, this volume).
The TOC concentration differences suggest that a greater fraction of the available carbon is consumed at Sites 899 and 900, which have slow accumulation rates, than at Sites 897 and 898. Although concentrations are variable at each site, averaged concentrations are lower at Sites 899 and 900 than they are at Sites 897 and 898. This difference is consistent with more efficient and therefore more complete consumption of organic matter under oxic conditions as opposed to anoxic conditions. Preservation of organic carbon is consequently poorer in the more slowly accumulated sediments at Sites 899 and 900.
Atomic C/N ratios help to distinguish between algal and land-plant origins of sedimentary organic matter. Algae typically have atomic C/N ratios between 4 and 10, whereas vascular land plants have C/N ratios of 20 and greater (Premuzic et al., 1982; Jasper and Gagosian, 1990; Meyers, 1994; Prahl et al., 1994). This distinction arises from the absence of cellulose in algae and its abundance in vascular plants and the consequent relative richness of proteinaceous material in algae, and it is largely preserved in sedimentary organic matter.
The averaged shorebased atomic C/N ratios of the Unit I samples are as follows: Site
897, 4.6
![]() 1.6; Site 898, 3.8
1.6; Site 898, 3.8 ![]() 3.9; Site 899, 3.7
3.9; Site 899, 3.7 ![]() 1.8; Site 900, 2.9
1.8; Site 900, 2.9 ![]() 2.1 (Meyers and Silliman,
this volume). These ratios indicate that marine contributions dominate the organic matter in these turbidite layers and that the organic matter has been at least partially degraded within its host sediment. Fresh algal organic matter typically has C/N ratios between 4 and 10 (Meyers, 1994). Degradation of organic matter in organic-carbon-poor marine sediments tends to lower C/N ratios as nitrogenous compounds break down to produce ammonia, which is retained by clay minerals, and the CO2 released by oxidation of organic carbon escapes (Müller, 1977). Some of the samples have C/N ratios of 1 or 2, too low to represent undegraded organic matter. A few samples have C/N ratios between 10 and 20; these samples probably contain elevated proportions of land-derived organic matter. Furthermore, the higher average C/N ratio of 4.6 in the Site 897 Unit I samples suggests that the marine organic matter at this location has experienced less degradation than has occurred at the other sites. Variation in the degree of preservation of marine-derived organic matter is suggested by the differences in C/N ratios from site to site and with depth at each site.
2.1 (Meyers and Silliman,
this volume). These ratios indicate that marine contributions dominate the organic matter in these turbidite layers and that the organic matter has been at least partially degraded within its host sediment. Fresh algal organic matter typically has C/N ratios between 4 and 10 (Meyers, 1994). Degradation of organic matter in organic-carbon-poor marine sediments tends to lower C/N ratios as nitrogenous compounds break down to produce ammonia, which is retained by clay minerals, and the CO2 released by oxidation of organic carbon escapes (Müller, 1977). Some of the samples have C/N ratios of 1 or 2, too low to represent undegraded organic matter. A few samples have C/N ratios between 10 and 20; these samples probably contain elevated proportions of land-derived organic matter. Furthermore, the higher average C/N ratio of 4.6 in the Site 897 Unit I samples suggests that the marine organic matter at this location has experienced less degradation than has occurred at the other sites. Variation in the degree of preservation of marine-derived organic matter is suggested by the differences in C/N ratios from site to site and with depth at each site.
Organic carbon isotopic ratios are useful to distinguish between marine and continental plant sources of sedimentary organic matter. Most photosynthetic plants incorporate carbon into organic matter using the C3 Calvin pathway, which biochemically discriminates against 13C to produce a  13C shift of about -20
13C shift of about -20 from the isotope ratio of the inorganic carbon source. Organic matter produced from atmospheric CO2 (
from the isotope ratio of the inorganic carbon source. Organic matter produced from atmospheric CO2 ( 13C
13C 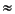 –7
–7 ) by land plants using the C3 pathway consequently has an average
) by land plants using the C3 pathway consequently has an average  13C (PDB) value of about -27
13C (PDB) value of about -27 (cf. O'Leary, 1988). The source of inorganic carbon for marine algae is dissolved bicarbonate, which has a
(cf. O'Leary, 1988). The source of inorganic carbon for marine algae is dissolved bicarbonate, which has a  13C
value of about 0
13C
value of about 0 . Marine organic matter consequently typically has
. Marine organic matter consequently typically has  13C values between -20
13C values between -20 and -22
and -22 . The isotopic difference between organic carbon produced by C3 land plants and marine algae has been used to trace the delivery and distribution of organic matter to sediments of ocean margins (Newman et al., 1973; Prahl et al., 1994). Carbon isotope ratios can be affected, however, by photosynthetic dynamics and by postdepositional diagenesis (Dean et al., 1986; McArthur et al., 1992) and consequently must be interpreted cautiously. Prominent among the factors that can impact
. The isotopic difference between organic carbon produced by C3 land plants and marine algae has been used to trace the delivery and distribution of organic matter to sediments of ocean margins (Newman et al., 1973; Prahl et al., 1994). Carbon isotope ratios can be affected, however, by photosynthetic dynamics and by postdepositional diagenesis (Dean et al., 1986; McArthur et al., 1992) and consequently must be interpreted cautiously. Prominent among the factors that can impact  13C values are the availability of CO2 during photosynthesis and the possibility of selective diagenesis of organic matter fractions that are isotopically heavy or light. Any diagenetic isotope shift appears to be small, less than 2
13C values are the availability of CO2 during photosynthesis and the possibility of selective diagenesis of organic matter fractions that are isotopically heavy or light. Any diagenetic isotope shift appears to be small, less than 2 (Hayes et al., 1989; Fontugne and Calvert, 1992; McArthur et al., 1992; Meyers, 1994). Increased availability of dissolved CO2 to algae, however, would enhance their isotopic discrimination and produce marine organic matter that is isotopically light (Hayes et al., 1989), as would increased delivery of isotopically light fluvial dissolved inorganic carbon (Fontugne and Calvert, 1992) from areas on the continental shelf where some of the turbidite-carried organic matter may have originated.
(Hayes et al., 1989; Fontugne and Calvert, 1992; McArthur et al., 1992; Meyers, 1994). Increased availability of dissolved CO2 to algae, however, would enhance their isotopic discrimination and produce marine organic matter that is isotopically light (Hayes et al., 1989), as would increased delivery of isotopically light fluvial dissolved inorganic carbon (Fontugne and Calvert, 1992) from areas on the continental shelf where some of the turbidite-carried organic matter may have originated.
Organic  13C values of Unit I sediment samples from the four sites average about -23
13C values of Unit I sediment samples from the four sites average about -23 , a value that indicates that most of the organic matter originated from marine production (Meyers, 1994). The range of these values, from -19.0
, a value that indicates that most of the organic matter originated from marine production (Meyers, 1994). The range of these values, from -19.0 to -26.8
to -26.8 (Meyers and Silliman, this volume), also suggests that the origins have not been identical and that the proportion of land-derived material may be important in some turbidite layers. The isotopically heavy organic matter may have been produced by marine algae during times of diminished availability of dissolved CO2, such as those that accompany spring blooms or upwelling. This organic matter may have originated from coastal areas of high productivity 200 km to the east of the abyssal plain and experienced transport to the deep-sea by turbidity flows of continental shelf sediment.
(Meyers and Silliman, this volume), also suggests that the origins have not been identical and that the proportion of land-derived material may be important in some turbidite layers. The isotopically heavy organic matter may have been produced by marine algae during times of diminished availability of dissolved CO2, such as those that accompany spring blooms or upwelling. This organic matter may have originated from coastal areas of high productivity 200 km to the east of the abyssal plain and experienced transport to the deep-sea by turbidity flows of continental shelf sediment.
Rock-Eval pyrolysis was originally developed to characterize the organic matter that is present in oil source rocks (Espitalié et al., 1977) and that is typically is more thermally mature and at higher concentrations than is the organic matter commonly found in sediments obtained by scientific ocean drilling. Rock-Eval analyses have nonetheless proved valuable in helping to determine organic matter sources in DSDP and ODP samples. Land-plant organic matter is usually rich in woody components and consequently has lower hydrogen indices (HI, defined as mg hydrocarbons per gram TOC) and higher oxygen indices (OI, defined as mg CO2 per gram TOC) than found in lipid-rich and cellulose-poor algal organic matter. This distinction between organic matter from continental and marine sources becomes blurred by diagenesis as marine matter oxidizes and gradually takes on HI and OI values similar to those of land-plant material.
A Van Krevelen-type plot of the HI and OI values suggests that the Leg 149 turbiditic sediments contain Type III (land-derived) organic matter (Fig. 7). This source assignment for the organic matter conflicts, however, with the low C/N ratios and the  13C values for these samples, both of which suggest that the organic matter is predominantly marine. The contradiction between the Rock-Eval source characterization and the elemental and isotopic source characterizations indicates that the original Type II (marine) organic matter has been heavily oxidized, probably by microbial reworking. The microbial reworking of the organic matter in the turbiditic units at Sites 899 and 900 evidently happened prior to, or shortly after, redeposition of these sediments on the Iberia Abyssal Plain, inasmuch as little sulfate reduction has occurred at these locations (Fig. 5, Fig. 6). In contrast, in situ microbial reprocessing of organic matter deeper below the sediment/water interface is indicated at Sites 897 and 898 by the disappearance of interstitial sulfate and accompanying appearance of pore- water methane with increasing sediment depth at these locations (Fig. 3, Fig. 4). The HI values of samples from Sites 897 and 898 are in general higher than those of samples from Sites 899 and 900 (Fig. 7), indicating that the marine organic matter at Sites 897 and 898 remains not as severely microbially reworked as at Sites 899 and 900.
13C values for these samples, both of which suggest that the organic matter is predominantly marine. The contradiction between the Rock-Eval source characterization and the elemental and isotopic source characterizations indicates that the original Type II (marine) organic matter has been heavily oxidized, probably by microbial reworking. The microbial reworking of the organic matter in the turbiditic units at Sites 899 and 900 evidently happened prior to, or shortly after, redeposition of these sediments on the Iberia Abyssal Plain, inasmuch as little sulfate reduction has occurred at these locations (Fig. 5, Fig. 6). In contrast, in situ microbial reprocessing of organic matter deeper below the sediment/water interface is indicated at Sites 897 and 898 by the disappearance of interstitial sulfate and accompanying appearance of pore- water methane with increasing sediment depth at these locations (Fig. 3, Fig. 4). The HI values of samples from Sites 897 and 898 are in general higher than those of samples from Sites 899 and 900 (Fig. 7), indicating that the marine organic matter at Sites 897 and 898 remains not as severely microbially reworked as at Sites 899 and 900.
Microbial oxidation of marine organic matter ( 13C values between -20
13C values between -20 and -22
and -22 ) can produce isotopically light dissolved inorganic carbon in the pore waters of deep-sea sediments (von Breymann et al., 1991). The remineralized CO2 can reprecipitate as carbonate and produce calcium carbonate with
) can produce isotopically light dissolved inorganic carbon in the pore waters of deep-sea sediments (von Breymann et al., 1991). The remineralized CO2 can reprecipitate as carbonate and produce calcium carbonate with  13C values as light as -19
13C values as light as -19 (Meyers and Snowdon, 1993). Because of the evidence for significant amounts of in situ organic matter degradation in the sediments of Sites 897 and 898, the CaCO3 carbon and oxygen stable isotopic contents were determined by the Stable Isotope Laboratory at The University of Michigan to investigate whether CO2 derived from organic matter was contributing to CaCO3 precipitation.
(Meyers and Snowdon, 1993). Because of the evidence for significant amounts of in situ organic matter degradation in the sediments of Sites 897 and 898, the CaCO3 carbon and oxygen stable isotopic contents were determined by the Stable Isotope Laboratory at The University of Michigan to investigate whether CO2 derived from organic matter was contributing to CaCO3 precipitation.
Nearly all of the carbonate  13C values are within the normal marine values from -1
13C values are within the normal marine values from -1 to +1
to +1 (Fig. 3, Fig. 4). Several excursions to
(Fig. 3, Fig. 4). Several excursions to  13C values as light as -4.5
13C values as light as -4.5 occur. Although these values might reflect a diagenetic contribution of CO2 derived from organic matter into CaCO3, two lines of evidence suggest that the isotopic values record initial conditions of carbonate formation at the sources of these turbidite components. First, the light
occur. Although these values might reflect a diagenetic contribution of CO2 derived from organic matter into CaCO3, two lines of evidence suggest that the isotopic values record initial conditions of carbonate formation at the sources of these turbidite components. First, the light  13C values consistently occur in sediment samples that contain less than 20% CaCO3 (Fig. 3, Fig. 4), which typically are elastics-dominated turbidite layers (Shipboard Scientific Party, 1994a, b). Diagenetic enrichment of the carbonate contents of these samples is not evident. Second,
13C values consistently occur in sediment samples that contain less than 20% CaCO3 (Fig. 3, Fig. 4), which typically are elastics-dominated turbidite layers (Shipboard Scientific Party, 1994a, b). Diagenetic enrichment of the carbonate contents of these samples is not evident. Second,  18O excursions to lighter values coincide with the
18O excursions to lighter values coincide with the  13C excursions. The general correspondence between
13C excursions. The general correspondence between  18O and
18O and  13C patterns in the sediments of both Site 897 and Site 898 (Fig. 3, Fig. 4) suggests that the isotopic content of seawater at the place of initial CaCO3 formation has controlled the sedimentary isotopic compositions. Moreover, the agreements in excursions to lighter values may record episodes of greater delivery of continental runoff, which is typically isotopically light in both carbon and oxygen, to areas on the continental margin of Iberia where the turbidites originated. Similar excursions to lighter isotopic values have been documented in Mediterranean sapropels by Fontugne and Calvert (1992), who postulated that the excursions record periods of enhanced freshwater delivery to this sea during the Pleistocene.
13C patterns in the sediments of both Site 897 and Site 898 (Fig. 3, Fig. 4) suggests that the isotopic content of seawater at the place of initial CaCO3 formation has controlled the sedimentary isotopic compositions. Moreover, the agreements in excursions to lighter values may record episodes of greater delivery of continental runoff, which is typically isotopically light in both carbon and oxygen, to areas on the continental margin of Iberia where the turbidites originated. Similar excursions to lighter isotopic values have been documented in Mediterranean sapropels by Fontugne and Calvert (1992), who postulated that the excursions record periods of enhanced freshwater delivery to this sea during the Pleistocene.
Concentrations of pore-water sulfate decrease nearly to zero in the upper 50 m of sediment at Sites 897 and 898 and remain near zero throughout the rest of Unit I at both sites (Fig. 3, Fig. 4). The decrease indicates an active zone of sulfate reduction at both sites. Below Unit I (292 mbsf at Site 897; 162 mbsf at Site 898), sulfate concentrations recover to 15 to 18 mM. Sulfate reduction appears not to occur in these deeper sediments. In terms of redox conditions, most of Unit I at Sites 897 and 898 is an anoxic section bounded by an upper and a lower sulfate reservoir.
Concentrations of pore-water sulfate in sediments of Sites 899 and 900 contrast to the pattern present in Sites 897 and 898 by progressively decreasing from near-seawater values (29 mM) as sediment depth increases (Fig. 5, Fig. 6). At least 15 mM interstitial sulfate is present in all parts of Unit I at these two sites.
The activity of sulfate-reducing bacteria is generally limited by the availability of short-chain organic compounds. As a result, organic matter degradation via sulfate reduction must be preceded by an intermediate fermentation that can break down organic compounds into smaller molecules. Organic matter at Sites 897 and 898 has slightly higher C/N ratios than does the organic matter at Sites 899 and 900 (Meyers and Silliman, this volume), which could be an indicator of better preservation of organic matter in these sediments. The high accumulation rates at Sites 897 and 898 evidently impacted subsequent microbial activity.
Concentrations of headspace methane are high in Unit I sediments from Sites 897 and 898 and drop to near-background levels in deeper sediments (Fig. 3, Fig. 4). Two sources of the gas are possible. First, gas from some deeper origin may have migrated into the unit, which consists of turbiditic sand, silt, and clay layers. The location of Sites 897 and 898 on basement highs makes this an especially reasonable possibility (Fig. 2). Evidence for migration of methane into porous sediments from deeper sources has been found at Sites 762 and 763 on the Exmouth Plateau where a known thermogenic source exists in underlying Jurassic rocks (Snowdon and Meyers, 1992). A similar deeper source for the methane on the Iberia Abyssal Plain is presently unknown. A second possibility is in situ formation by methanogenic bacteria. High methane/ethane ratios and the absence of higher-molecular-weight hydrocarbon gases indicate that the gas is biogenic, as opposed to thermogenic, in origin (Shipboard Scientific Party, 1994a, b). The source of the methane may be from in situ microbial fermentation of the marine organic matter present in this turbiditic unit. Similar microbial production of methane from marine organic matter has been inferred from high biogenic gas concentrations in Pliocene- Pleistocene sediments from Site 532 on the Walvis Ridge (Meyers and Brassell, 1985) and in middle Miocene sediments from Site 767 in the Celebes Sea (Shipboard Scientific Party, 1990).
Headspace methane concentrations are low in sediments below Unit I at Sites 897 and 898 (Fig. 3, Fig. 4). Sub-bottom depths of 200 to 300 m are not likely to be important in limiting microbial methane production inasmuch as viable heterotrophic bacteria have been isolated from upper Miocene sediments more than 500 meters below the seafloor at Site 798 on the Oki Ridge (Cragg et al., 1992). The lower amounts of metabolizable organic matter in deeper sediments is probably the major factor in precluding methanogenesis. The presence of significant concentrations of pore-water sulfate (Fig. 3, Fig. 4) indicates that methanogenesis is not energetically favored in sediments deeper than Unit I (Claypool and Kvenvolden, 1983).
In contrast to Sites 897 and 898, the sediments of Sites 899 and 900 contain essentially no methane (Fig. 5, Fig. 6). Because methane is present at sub-bottom depths at Sites 897 and 898 that are similar to those at Sites 899 and 900, migration out of the sediments is unlikely. Instead, it is probable that methane was never generated in the sediments at Sites 899 and 900. Organic carbon concentrations are, on average, lower at these two sites than they are at Sites 897 and 898, and the organic matter present in the sediments appears to be detrital and of low metabolizable value (Meyers and Silliman, this volume). The consequent absence of suitable substrate for microbial activity may have prevented methanogenesis. In addition, interstitial sulfate is present at near-seawater concentrations in the sediments of Sites 899 and 900 (Fig. 5, Fig. 6), and this may have imposed a further constraint on methanogenic bacterial activity (Claypool and Kvenvolden, 1983). Alternatively, the presence of significant amounts of dissolved sulfate may reflect the absence of much microbial activity at Sites 899 and 900.