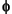 (
(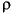 z) procedure) supplied by the manufacturers (Pouchou and Pichoir, 1985).
z) procedure) supplied by the manufacturers (Pouchou and Pichoir, 1985).
To characterize the mineral chemistry associated with different textures described, we selected 15 samples for microprobe analyses (see Fig. 2 for their location in the basement section). The samples were analyzed in the electron microprobe Cameca SX50 at the University of Granada. An acceleration voltage of 15 kV, beam current of 20 nA, and beam diameter of 8 µm were used. Standards were simple synthetic oxides (Al2O3, Fe2O3, Cr2O3, MnTiO4, and MgO) and natural silicates (albite, orthoclase, wollastonite), and sphalerite. Data were reduced using the PAP procedure (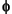 (
(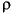 z) procedure) supplied by the manufacturers (Pouchou and Pichoir, 1985).
z) procedure) supplied by the manufacturers (Pouchou and Pichoir, 1985).
In this paper, we present the main characteristics of the mineral chemistry data for every major mineral phase, and combine the data from high-grade schist with those from pelitic and migmatite gneiss.
Garnet porphyroblasts have a large chemical variation (Fig. 3, Fig. 4; Table 1). Metapelite garnets usually have variable Fe, Ca, and Mn contents, with a low and constant Mg content. Garnets from gneissic rocks are very close in composition to those from the high-grade schist. (Because we have normalized all the garnet analyses to a mineral chemical formula with 12 oxygens and Fetotal = Fe2+ [see Table 1], all the iron contents described in this paper should be considered as the sum of almandine and andradite end-member contents.)
The two garnet types distinguished in the staurolite-bearing high-grade schist each have distinctive zoning pattern. Garnet I porphyroblasts have a normal zoning pattern indicating prograde growth: decreasing spessartine and increasing almandine content from core to rim (Alm0.65-0.80 Grs0.05-0.20 Pyr0.02-0.06 Sps0.05-0.13; Fig. 3A, Fig. 4A). Garnet II rims and grains have a fairly constant composition (Fig. 3B, Fig. 4B), similar to the rims of garnet I, but with some sharp increases in spessartine and grossular at the rims (i.e., inverse zoning patterns; up to Alm0.62 Grs0.22 Pyr0.04 Sps0.12). Incomplete or truncated zoning patterns are present in grains included in andalusite porphyroblasts (garnet II) and partially corroded garnet I grains.
Garnets from corundum-bearing schist have a higher grossular content (Grs0.20-0.35) and a low spessartine content (Sps0.12-0.15), with low pyrope (Pyr0.05-0.08). These garnets have a normal zoning pattern, characterized by increasing almandine content and decreasing spessartine content from core to rim (Fig. 3C). Grossular content decreases toward the rims (Fig. 4C).
Garnet in gneiss has two distinctive textures and chemical compositions (Fig. 3E, Fig. 4E). Relict and corroded garnet I porphyroblasts have a large chemical variation (Alm0.72-0.81 Grs0.07-0.10 Pyr0.06 Sps0.06-0.15), similar to the zoning pattern of garnet I from high-grade schist. Garnet II porphyroblasts have reverse zoning patterns, characterized by high and increasing spessartine content (Sps0.10-0.16), decreasing almandine content (Alm0.82-0.76) toward the rims, and low and constant abundance of grossular and pyrope (Grs0.03 Pyr0.04).
The compositional range of plagioclase in all rock types is presented in Figure 5 and Table 2. Plagioclase in high-grade schist has high to medium An contents (especially in corundum-bearing rocks; XAn = 0.95-0.60). In gneissic rocks, in contrast, plagioclase has moderate An content, within a small variational range (XAn = 0.50-0.35), similar to the Ab-rich composition of plagioclase in granite rocks (XAn = 0.40-0.30).
Plagioclase porphyroblasts in high-grade schist have variable zoning patterns, with oscillatory and normal zoning (i.e., more albite-rich toward rims) as the most common types. Some matrix porphyroblasts have a thin outermost rim of K-feldspar (XAb = 0.05-0.15); surrounding plagioclase II rims and with inclusions of fibrolite and biotite. The composition of plagioclase inclusions spans from XAn = 0.4-0.6, in garnet II porphyroblasts, to XAn = 0.8 in the outer rim of garnet I grains (Fig. 5A).
We have observed K-feldspar (XAb = 0.02-0.08) with plagioclase (XAn = 0.50-0.64) intergrowths included in the rims of garnet II porphyroblasts. This texture could be interpreted as coexisting feldspars crystallizing during subsolidus cooling, rather than as exsolution textures.
Plagioclase in leucosome veins (XAn = 0.38-0.44) and granite rocks (XAn = 0.30-0.44) has flat zoning patterns, with lower An content. Some plagioclase phenocrysts have a moderate increase in albite in the outermost rim, which can be partially surrounded by K-feldspar. Plagioclase in pelitic domains of the gneiss, however, has compositions close to those from high-grade schist (XAn = 0.50-0.58), also suggesting that it is restitic.
Biotite has a large range in composition (see Table 3), as shown in the "ideal biotite plane" diagram of Guidotti (1984; Fig. 6). It varies between the biotite end-members annite (Al-poor, Fe-rich biotite) and siderophyllite (Al- and Fe-rich biotite). In corundum-bearing schist and gneiss, biotite tends toward the Al-rich phlogopite composition (eastonite). It is important to note that these have high-Al content (Al[vi] ~ 1.0 ions per formula unit [pfu]), with up to 1.3 ions pfu. These values are outside the compositional range found in natural metamorphic biotites (see Guidotti, 1984) and could be interpreted as the result of disequilibrium relationships in these rocks.
In gneiss, biotite has two distinctive compositions (Fig. 6). First, biotite from pelitic domains has a composition toward Al-rich annite [mg# ~ 0.25; mg# = Mg/(Mg + Fe)]. In contrast, biotite in leucosome domains is closer to phlogopite compositions (mg# ~ 0.4-0.5). This difference indicates that biotite grew during two separate metamorphic stages.
High Ti content characterizes biotite grains in all the rock types (0.3-0.45 ions pfu), with the exception of corundum-bearing metapelites, where biotite has low Ti content (< 0.2 ions pfu). Al[vi] content varies widely from staurolite-bearing schist (0.70-1.1 ions pfu) to gneissic rocks (0.79-1.0 ions pfu), and granite rocks (0.86-1.34 ions pfu). Mg content increases in biotite from banded gneiss and pelitic domains in migmatite gneiss (1.0-1.75 ions pfu; mg# = 0.27-0.37); to leucosome veins (>2.25 ions pfu; mg# = 0.49-0.61). Biotite from high-grade schists has low Mg content in any textural position (0.75-1.25 ions pfu; mg# = 0.16-0.25).
In conclusion, matrix porphyroblasts in all the rock types show a homogeneous composition largely controlled by bulk-rock chemistry variations, apart from those biotite grains close to destabilization minerals (e.g., garnet, staurolite, cordierite, etc.).
Staurolite has a little chemical variation in Fe, which is higher in matrix porphyroblasts (2.9-3.1 ions pfu) than in relict grains included in andalusite (2.6-2.8 ions pfu; Table 4). Mg and Zn contents are always very low (<0.2 ions pfu and <0.17, respectively). Relict inclusions of staurolite in garnet II from the high-grade schist usually have a typical increase of the Mg:Fe ratio with respect to matrix porphyroblasts. This variation suggests that these staurolite grains are involved in the garnet-forming reactions.
Staurolite is partially transformed to hercynite (Hc0.9 Spl0.1; Table 4) inside andalusite porphyroblasts and to ilmenite and pyrite in the matrix.
Cordierite, either as relict or partially pinnitized grains porphyroblasts in the matrix, has a constant chemical composition, with Mg/(Mg + Fe + Mn) values between 0.6 and 0.7 (Table 4). The total amount of oxides in nontransformed cordierite grains (98-98.5 wt%) suggests that this mineral could have up to 1.5-2.0 wt% H2O.
Magnetite and Ti-magnetite are present exclusively in gneissic rocks (Table 4). The latter is preserved only inside biotite porphyroblasts. The presence of Ti-magnetite has been previously reported in other high-grade rocks (see Rumble, 1976), as this mineral is restricted to temperatures higher than 600°C (Lindsley, 1976). (Although the term Ti-magnetite should be restricted to those specimens where an ulvöspinel phase can be demonstrated by X-ray analysis [Deer et al., 1966], we have used this term to indicate the occurrence of a significant Ti = Fe3+ substitution in magnetite.)