RESULTS AND DISCUSSION
The results of elemental analysis for total organic carbon (TOC) and carbonate content in the two investigated sequences across organic-matter-rich layers in the Alboran Basin Hole 161-977A are compiled in Table 1 and
Figure 3. The organic carbon contents in Sections 161-977A-18X-5 and -6 vary between 0.74% and 1.90% while in Section 977A-9H-5 they range from 0.88% to 2.27%. The more pronounced symmetric form of the TOC profile in the samples from Section 977A-9H-5 and the more regular sample spacing led us to investigate this sequence in greater detail on a molecular level. TOC content of organic-matter-rich layers in the Western Mediterranean Sea are distinctly lower than those in eastern Mediterranean sapropels, which, considering the proximity to the African and Spanish coasts, may mainly reflect a higher supply of clastic material from the continent and thus a higher degree of terrigenous dilution, but may also be a factor of lower primary productivity and inferior conditions for organic matter preservation in the Alboran Basin. In the background sediments below and above the organic-matter-rich layers, recovered in the Alboran Sea during Leg 161, the average TOC content is more than twice as high as the deep-ocean sediment average of 0.3% reported by McIver (1975) and distinctly higher than in nonsapropel background sediment of the Eastern Mediterranean Sea (Emeis, Robertson, Richter, et al., 1996). Maximum TOC values at the four sites occupied during Leg 161 in the Alboran Sea are in the range of 1.27% (Site 978) to 2.50% (Site 977; Comas, Zahn, Klaus, et al., 1996). The carbonate contents of the investigated samples are between 20%-50% and indicate varying influences of bioproductivity, dilution by noncarbonate material and post-depositional carbonate solution; the carbonate data are positively correlated with the TOC values.
Six of the ten samples from Section 161-977A-9H-5 (marked in Fig. 3) were selected for further molecular organic geochemical investigations. The sterols are quantitatively the most important group of compounds among those separated by liquid chromatography and analyzed quantitatively, while the n-aldehydes represent the least abundant group (Fig. 4). The data in Figure 4 represent the sum of concentrations of identifiable single compounds (together making up 4% to 9% of the total extracts), but are not identical to the amounts of total liquid chromatography fractions separated from the total extracts. Among the different compound groups, only the n-alkanes correlate positively with the TOC values of the entire interval studied. The long-chain n-alkenones, n-alkanols, fatty acids, and squalene have a significant maximum in the 86- to 88-cm interval of Section 161-977A-9H-5, while the n-alkanolones and diols have a minimum there. The ketols and diols show a distinct positive correlation with each other (R = 0.97).
n-Alkanes
The n-alkanes in the nonaromatic hydrocarbon fraction of the extracts cover a range of chain lengths between C17 and C40 (Fig. 5). The C23 and C35 n-alkanes dominate and exhibit a pronounced odd-over-even carbon-number preference typical of an origin of terrestrial higher plants (Eglinton et al., 1962). The n-alkanes between C15 and C23 have a low abundance and do not exhibit a characteristic pattern of an intense autochthonous supply of phytoplanktonic organic matter (Blumer et al., 1971). The distribution without any preference may be due to co-occurring phyto- and zooplankton signals, with the even-over-odd carbon-number preference of the latter (Giger and Schaffner, 1977) being superimposed on the odd-over-even carbon-number predominance of the phytoplankton (Blumer et al., 1971), but the broad and smooth distribution of short-chain n-alkanes may also be a result of the presence of a certain amount of recycled, more mature organic matter commonly found in deep-sea sediments (e.g., Tissot et al., 1980; Summerhayes, 1981). The n-alkanes with chain lengths beyond C36 may be a result of the incorporation of coccolithophore biomass into the sediments (Volkman et al., 1980c), which is likely to be also responsible for the presence of the long-chain n-alkenones (Volkman et al., 1980b).
Another aspect of the n-alkane distributions is the unexpectedly small variation of their distribution pattern over the organic-matter-rich layer. Together with the fact that organic-carbon-normalized concentrations of n-alkanes vary little, that is, they increase with the increase of total organic carbon, this shows that deposition of the organic-matter-rich layer is accompanied by an increase in the supply of terrigenous organic matter to the Alboran Basin.
Fatty Acids
While the n-alkanes show mainly a terrestrial influence, the fatty acids are dominated by short-chain marine or microbial components. The chain length of the fatty acids ranges from C11 to C30 with a distinct maximum at C16 and a strong even-over-odd carbon number predominance (Fig. 6). Diatoms may be the main contributors of the fatty acids in the Alboran basin sediments because they mainly produce C16 and C18 homologs (Volkman et al., 1980a). Dinoflagellates mainly biosynthesize C18, C20, and C22 fatty acids (Harrington et al., 1970), while coccolithophores have C14 and C16 (Volkman et al., 1981), green algae C16 and C18 (Patterson, 1970), blue-green algae C14, C16, and C18 acids (Kenyon et al., 1972).
n-Alkanols
The n-alkanols exhibit a mainly terrestrial carbon number distribution pattern (Fig. 7). The chain lengths range from C12 to C32 with a predominance of the higher-carbon-number homologs and a strong even-over-odd carbon-number preference. Noteworthy is the depth profile of the C22 n-alkanol. Algal mats have been reported to contain this compound as a major constituent (Cardoso et al., 1976), but the strong correlation with the long-chain fatty acids in the studied interval from the Alboran Basin (R = 0.95; Fig. 8) favors a common terrestrial source of both the C22 alcohol and the long-chain fatty acids, possibly initially ester-linked.
Ketols and Diols, Long-Chain Alkenones
The sum of the concentrations of 1-hydroxytriacontan-15-one and 1-hydroxydotriacontan-15-one and the sum of the concentrations of all long-chain diols (Table 2) correlate strongly with each other over depth, while both classes apparently do not have any relationship with the long-chain alkenones (Fig. 9). This lack of correlation of the long-chain alkenones with the other two compound classes corroborates earlier reports of different sources. Prymnesiophytes, and particularly coccolithophores, are well known as producers of heptatriaconta-8(E),15(E),22(E)-triene and heptatriaconta-15(E),22(E)-diene as well as other long-chain alkenones and alkenoates (Prahl and Wakeham, 1987). The source of the ketols and diols has been discussed controversially for a long time. While Morris and Brassell (1988) suggested cyanobacteria as a probable source, Volkman et al. (1992) identified specific marine green algae (Eustigmatophytes) as a more likely source of these compounds. The suggestion of de Leeuw et al. (1981) that coccolithophores may be the source of ketols and diols as of the n-alkenones could not be confirmed here.
Paleo-sea-surface temperatures based on the ratio of di- and tri-unsaturated long-chain ketones (Prahl and Wakeham, 1987) show a distinct increase from 19°C to 22°C over the short sediment interval studied here, with the main increase occurring after the deposition of the organic-matter-rich layer (Fig. 10).
Pentacyclic Triterpenoid Ketones
The concentrations of two angiosperm pentacyclic triterpenoid ketones, lup-20(29)-en-3-one and olean-12-en-3-one, closely correlate with each other (R = 0.98). In contrast to this, the concentration of friedelan-3-one has a different depth profile, with a decrease toward the top of the studied section. This is taken as evidence of different taxonomic sources of friedelan-3-one and the other two triterpenoid ketones. As a terrestrial biomarker friedelan-3-one, other than lup-20(29)-en-3-one and olean-12-en-3-one, is mostly associated with monocotyledons (Das and Mahato, 1983). Monocotyledons in general prefer open, thinly vegetated areas and are more robust under rough climatic conditions. The decreasing upward trend of friedelan-3-one concentration may indicate a climatic change to higher temperatures and increasingly dense wood vegetation on land during the deposition of the sediment interval studied. This would be consistent with the temperature trend calculated from the alkenone index.
Sterols
More than 50 different free sterols were detected and quantified (Table 3, Table 4). Among them, cholesterol (i), 5 -cholestan-3ß-ol (j), 24-methylcholesta-5,22(E)-dien-3ß-ol (A), 24-methyl-5
-cholestan-3ß-ol (j), 24-methylcholesta-5,22(E)-dien-3ß-ol (A), 24-methyl-5 (H)-cholest-22 (E)-en-3ß-ol (B), 24-ethylcholest-5-en-3ß-ol (M´), 23,24-dimethyl-5
(H)-cholest-22 (E)-en-3ß-ol (B), 24-ethylcholest-5-en-3ß-ol (M´), 23,24-dimethyl-5 (H)-cholestan-3ß-ol (N´; note that M´ and N´ coelute with fucosterol, L´, but the former two compounds represent the main part of the peak), and 24-ethyl-5
(H)-cholestan-3ß-ol (N´; note that M´ and N´ coelute with fucosterol, L´, but the former two compounds represent the main part of the peak), and 24-ethyl-5 (H)-cholestan-3ß-ol (P´) are the main free sterols in the six samples together with the 4-methylsterols 4
(H)-cholestan-3ß-ol (P´) are the main free sterols in the six samples together with the 4-methylsterols 4 ,24-dimethyl-5
,24-dimethyl-5 (H)-cholestan-3ß-ol (Q´; note that P´ and Q´ coelute with isofucosterol, R´, but the former two compounds represent the main part of the peak), dinosterol (C´´), and two isomers of 4
(H)-cholestan-3ß-ol (Q´; note that P´ and Q´ coelute with isofucosterol, R´, but the former two compounds represent the main part of the peak), dinosterol (C´´), and two isomers of 4 ,23,24-trimethyl-5
,23,24-trimethyl-5 (H)-cholestan-3ß-ol (H´´ and I´´).
(H)-cholestan-3ß-ol (H´´ and I´´).
Over the depth interval studied, the distribution patterns of the sterols exhibit a large variation (Table 4). The relative proportions of C28 and C30 sterols as a fraction of total sterols correlate with the TOC content, while the summed concentration of the C29 sterols steadily increases with decreasing sediment depth. The C27 sterol concentrations have an inverse relationship to that of the C29 sterols as a function of depth. This indicates an increase of terrestrial influence with time. The triangular Huang-Meinschein diagram of sterol carbon number distributions
(Fig. 11) illustrates this trend. This observation is in contrast, however, to the interpretations based on the homologous series of n-alkanes, n-fatty acids, and n-alcohols. They show a higher deposition of marine organic matter in the 63- to 65-cm interval. It is conceivable that the sterol carbon number assignment to marine and terrigenous sources of Huang and Meinschein (1976, 1979) does not strictly hold here. Except for 23,24-dimethyl-5 (H)-cholestan-3ß-ol (N´), all of the C28 and C29 sterols, which are the main sterols in the Alboran Sea sediments, have been shown to be produced also by marine organisms and to occur in sediments dominated by marine organic matter (Volkman, 1986). In particular, 24-ethyl-5
(H)-cholestan-3ß-ol (N´), all of the C28 and C29 sterols, which are the main sterols in the Alboran Sea sediments, have been shown to be produced also by marine organisms and to occur in sediments dominated by marine organic matter (Volkman, 1986). In particular, 24-ethyl-5 (H)-cholestan-3ß-ol (P´) may be produced in relatively large amounts by marine phytoplankton. In addition, the Huang-Meinschein diagram does not take into account the occurrence of C26 and C30 sterols, which are all from marine sources (Volkman, 1986). This is particularly true for the main 4-methyl C30 sterols; 4
(H)-cholestan-3ß-ol (P´) may be produced in relatively large amounts by marine phytoplankton. In addition, the Huang-Meinschein diagram does not take into account the occurrence of C26 and C30 sterols, which are all from marine sources (Volkman, 1986). This is particularly true for the main 4-methyl C30 sterols; 4 ,23,24- trimethyl-5
,23,24- trimethyl-5 (H)-cholest-22(E)-en-3ß-ol (dinosterol, C''), and 4
(H)-cholest-22(E)-en-3ß-ol (dinosterol, C''), and 4 ,23, 24-trimethyl-5
,23, 24-trimethyl-5 (H)-cholestan-3ß-ol (H´´ and I´´) are produced by dinoflagellates (Alam et al., 1979; Kokke et al., 1981). Dinoflagellates are also regarded as the main source of the other 4-methylsterols (de Leeuw et al., 1983). Based on this rationalization, the sterols in the Hole 977A sediments show an increasing marine bioproductivity during deposition of the organic-matter-rich layer, in addition to an obvious higher terrigenous inflow, as revealed by the n-alkane concentration profile.
(H)-cholestan-3ß-ol (H´´ and I´´) are produced by dinoflagellates (Alam et al., 1979; Kokke et al., 1981). Dinoflagellates are also regarded as the main source of the other 4-methylsterols (de Leeuw et al., 1983). Based on this rationalization, the sterols in the Hole 977A sediments show an increasing marine bioproductivity during deposition of the organic-matter-rich layer, in addition to an obvious higher terrigenous inflow, as revealed by the n-alkane concentration profile.
Lignin Phenols
Lignin is only produced by land plants except eulithoral grasses (Leo and Barghoorn, 1970; Quackenbush et al., 1986). Lignin may comprise 35% to 40% of the dry mass of wood (e.g., Beyer and Walter, 1981) and over 50% of the global primary production can be assigned to vascular plants (Olson et al., 1985; Martin et al., 1987). Moreover, only some fungi are able to degrade lignin, which renders it very stable over long periods of time (Hedges et al., 1988; Goñi et al., 1993). These three parameters, mass, ubiquity, and persistence, make lignin a suitable tracer for organic material of terrigenous origin.
The summed concentrations of the lignin phenols are usually termed 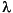 or
or 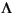 , where
, where 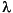 is defined as the sum of the vanillyl and syringyl phenols in mg/100 g TOC (Hedges and Parker, 1976), while
is defined as the sum of the vanillyl and syringyl phenols in mg/100 g TOC (Hedges and Parker, 1976), while 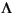 includes the cinnamyl phenols as well. The p-hydroxyphenols are not considered, because they can also come from marine sources. The lignin phenols show depth profiles similar to those of the straight-chain homologous series and the sterols, that is, during the deposition of the organic-matter-rich layer there is a slightly less allochthonous supply than before and after this time
(Fig. 12). It has to be noted, however, that the concentrations of lignin phenols measured in the Alboran Basin sediments are three orders of magnitude smaller than those reported for Holocene sediments by Hedges and Parker (1976) and Goñi and Hedges (1990); therefore, concentrations of
includes the cinnamyl phenols as well. The p-hydroxyphenols are not considered, because they can also come from marine sources. The lignin phenols show depth profiles similar to those of the straight-chain homologous series and the sterols, that is, during the deposition of the organic-matter-rich layer there is a slightly less allochthonous supply than before and after this time
(Fig. 12). It has to be noted, however, that the concentrations of lignin phenols measured in the Alboran Basin sediments are three orders of magnitude smaller than those reported for Holocene sediments by Hedges and Parker (1976) and Goñi and Hedges (1990); therefore, concentrations of 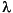 and
and 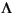 are in µg/g TOC here.
are in µg/g TOC here.

 -cholestan-3ß-ol (j), 24-methylcholesta-5,22(E)-dien-3ß-ol (A), 24-methyl-5
-cholestan-3ß-ol (j), 24-methylcholesta-5,22(E)-dien-3ß-ol (A), 24-methyl-5 (H)-cholest-22 (E)-en-3ß-ol (B), 24-ethylcholest-5-en-3ß-ol (M´), 23,24-dimethyl-5
(H)-cholest-22 (E)-en-3ß-ol (B), 24-ethylcholest-5-en-3ß-ol (M´), 23,24-dimethyl-5 (H)-cholestan-3ß-ol (N´; note that M´ and N´ coelute with fucosterol, L´, but the former two compounds represent the main part of the peak), and 24-ethyl-5
(H)-cholestan-3ß-ol (N´; note that M´ and N´ coelute with fucosterol, L´, but the former two compounds represent the main part of the peak), and 24-ethyl-5 (H)-cholestan-3ß-ol (P´) are the main free sterols in the six samples together with the 4-methylsterols 4
(H)-cholestan-3ß-ol (P´) are the main free sterols in the six samples together with the 4-methylsterols 4 ,24-dimethyl-5
,24-dimethyl-5 (H)-cholestan-3ß-ol (Q´; note that P´ and Q´ coelute with isofucosterol, R´, but the former two compounds represent the main part of the peak), dinosterol (C´´), and two isomers of 4
(H)-cholestan-3ß-ol (Q´; note that P´ and Q´ coelute with isofucosterol, R´, but the former two compounds represent the main part of the peak), dinosterol (C´´), and two isomers of 4 ,23,24-trimethyl-5
,23,24-trimethyl-5 (H)-cholestan-3ß-ol (H´´ and I´´).
(H)-cholestan-3ß-ol (H´´ and I´´).
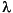 or
or 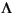 , where
, where