Pore-water data from the Blake Ridge (Fig. 1) were gathered from sediments collected by both ODP operations on Leg 164 and piston coring.
Procedures for collecting
and processing piston core sediments and their pore waters are described in
Borowski (1998). Sediments collected from ODP cores were sampled and processed
using standard ODP methods, except that pore-water samples were taken more
frequently at every ~1.5 m (1 sample per section, or 6 samples per core) (e.g., Fig.
3). Pore waters were extracted from sediments using a hydraulic sediment
press (Manheim, 1966) and collected in airtight syringes (Manheim and Sayles,
1974). Sulfate concentration was determined by ion chromatography, and
alkalinity was measured by titration (Gieskes et al., 1991). Total carbon
dioxide (![]() CO2)
was separated and measured onshore using cyrogenic and manometric techniques
(Craig, 1953) from pore-water aliquots stored immediately after squeezing in
flame-sealed, airtight ampoules. Methane concentrations within piston core and
Site 994 sediments were measured on shipboard using headspace methods (Martens
and Klump, 1980; Kvenvolden and McDonald, 1985; Borowski et al., 1997) and
normalized to pore space volume (Paull, Matsumoto, Wallace, et al., 1996).
Methane concentrations from Site 995 pore waters were measured from subcores
transported to an onshore laboratory, and normalized to pore space volume (Hoehler
et al., Chap. 8,
this volume).
CO2)
was separated and measured onshore using cyrogenic and manometric techniques
(Craig, 1953) from pore-water aliquots stored immediately after squeezing in
flame-sealed, airtight ampoules. Methane concentrations within piston core and
Site 994 sediments were measured on shipboard using headspace methods (Martens
and Klump, 1980; Kvenvolden and McDonald, 1985; Borowski et al., 1997) and
normalized to pore space volume (Paull, Matsumoto, Wallace, et al., 1996).
Methane concentrations from Site 995 pore waters were measured from subcores
transported to an onshore laboratory, and normalized to pore space volume (Hoehler
et al., Chap. 8,
this volume).
![]() CO2
was separated and purified using cryogenic techniques (e.g., Craig, 1953).
Carbon-isotopic-composition measurements of
CO2
was separated and purified using cryogenic techniques (e.g., Craig, 1953).
Carbon-isotopic-composition measurements of ![]() CO2
are reported relative to the Peedee belemnite (PDB) standard, and were made
using a Delta E mass spectrometer at North Carolina State University (NCSU). The
cumulative (vacuum line and mass spectrometer) accuracy and precision of
isotopic measurements are ±0.2
CO2
are reported relative to the Peedee belemnite (PDB) standard, and were made
using a Delta E mass spectrometer at North Carolina State University (NCSU). The
cumulative (vacuum line and mass spectrometer) accuracy and precision of
isotopic measurements are ±0.2![]() and ±0.06
and ±0.06![]() (Neal Blair, pers. comm., 1996).
(Neal Blair, pers. comm., 1996).
Sulfur isotopic
measurements of interstitial sulfate were made at Geochron Laboratories
(Cambridge, MA). Sulfate within pore waters was precipitated as barium sulfate,
thermally decomposed to SO3, and reduced to SO2 gas for
isotopic measurement (Holt and Engelkemeir, 1970). Measurements were made using
a VG Micromass mass spectrometer (Model 903), and are reported relative to the
Canyon Diablo Troilite (CDT) standard. Measurement precision is ±0.1![]() and cumulative accuracy is ±0.3
and cumulative accuracy is ±0.3![]() (Marshall Otter, pers. comm., 1997).
(Marshall Otter, pers. comm., 1997).
Variations in the concentration of interstitial constituents relative to time and depth can be described by equations relating the mass-transport processes of diffusion, advection (burial and compaction) to consumption and production reactions (e.g., Berner, 1980). The general diagenetic equation used here is
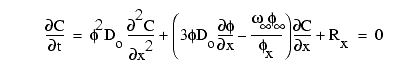 (3)
(3)
where C is concentration,
t is time, ![]() is porosity, Do
is the free-solution diffusion coefficient, x is depth below the sediment-water
interface,
is porosity, Do
is the free-solution diffusion coefficient, x is depth below the sediment-water
interface, ![]() is sedimentation or burial rate, and R is reaction rate (Berner, 1964, 1977).
The subscript (
is sedimentation or burial rate, and R is reaction rate (Berner, 1964, 1977).
The subscript (![]() )
refers to the depth where
)
refers to the depth where ![]()
![]() /
/![]() x
approaches zero. This form of the diagenetic equation was initially applied to
anoxic, methane-oxidizing sediments by Murray et al. (1978).
x
approaches zero. This form of the diagenetic equation was initially applied to
anoxic, methane-oxidizing sediments by Murray et al. (1978).
The model explicitly
assumes that: (1) steady state conditions occur (i.e., ![]() C/
C/![]() t
= 0); (2) vertical concentration gradients are much greater in magnitude than
horizontal gradients; (3) sediment burial, compaction, and molecular diffusion
are the only mass-transport processes (no fluid advection, bioirrigation, or gas
ebullition occurs); (4) the free-solution diffusion coefficient and sediment
density are constant with depth; and (5) the diffusion coefficient within
sediment (Ds) is related to free-solution diffusion coefficient by
the expression, Ds =
t
= 0); (2) vertical concentration gradients are much greater in magnitude than
horizontal gradients; (3) sediment burial, compaction, and molecular diffusion
are the only mass-transport processes (no fluid advection, bioirrigation, or gas
ebullition occurs); (4) the free-solution diffusion coefficient and sediment
density are constant with depth; and (5) the diffusion coefficient within
sediment (Ds) is related to free-solution diffusion coefficient by
the expression, Ds = ![]() 2
Do (Ullman and Aller, 1982).
2
Do (Ullman and Aller, 1982).
An inverse, numerical
model was used to predict reaction rates and their depth distribution using
Equation 3 by solving for Rx for data collected at Site 995 (for
model parameters see Table 1).
Cubic spline functions were used to fit methane concentration data providing the
necessary gradient information used to calculate the first and second derivative
terms of Equation 3. Cubic splines are particularly good for fitting purposes
because they: (1) have no inherent functional form (e.g., like exponential or
polynomial functions); (2) have continuous first and second derivatives; and (3)
can be modified to accept measurement uncertainties (Ahlberg et al., 1967;
Alperin, 1988; Alperin et al., 1988). Porosity data (Paull, Matsumoto, Wallace,
et al., 1996) were fit using an exponential function that provided ![]() x
and
x
and ![]()
![]() .
The free-solution coefficient (Do) used for methane was 7.9
.
The free-solution coefficient (Do) used for methane was 7.9 ![]() 10-6 cm2 s-1 (3șC; calculated from
empirical data given by Li and Gregory, 1974; Lerman, 1979), based on a sediment
temperature of ~4șC (Ruppel, 1997). The sedimentation rate was assumed to be
constant at 0.0057 cm y-1 (5.7 cm ky-1), based
on the last appearance of Pseudoemiliania lacunosa (Paull, Matsumoto,
Wallace, et al., 1996), which occurred at 0.46 Ma (Thierstein et al., 1977).
10-6 cm2 s-1 (3șC; calculated from
empirical data given by Li and Gregory, 1974; Lerman, 1979), based on a sediment
temperature of ~4șC (Ruppel, 1997). The sedimentation rate was assumed to be
constant at 0.0057 cm y-1 (5.7 cm ky-1), based
on the last appearance of Pseudoemiliania lacunosa (Paull, Matsumoto,
Wallace, et al., 1996), which occurred at 0.46 Ma (Thierstein et al., 1977).