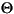 Fe(II) ~350 K and
Fe(II) ~350 K and 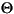 Fe(III) ~500 K (typical values for oxides, hydroxides, silicates, or carbonates; De Grave and Van Alboom, 1991), yields a systematic error of 1%.
Fe(III) ~500 K (typical values for oxides, hydroxides, silicates, or carbonates; De Grave and Van Alboom, 1991), yields a systematic error of 1%.
The investigation was conducted using materials collected during ODP Leg 172, which recovered a sequence of sediment cores along a depth transect in the westernmost North Atlantic to obtain a detailed history of late Neogene climate change. Eleven sites were cored on the Carolina Slope, along the Blake-Bahama Outer Ridge, on the Bermuda Rise, and on the Sohm Abyssal Plain. Hole 1062A (30°45.6'N, 74°28.0'W) was retrieved at a water depth of 4763 m on the eastern flank of a mud wave, located in a mud wave field in the Bahama Basin (Shipboard Scientific Party, 1998a).
For our study, 12 samples (three samples per core from Cores 172-1062A-10H through 13H) were taken during the cruise shortly after opening the cores. The samples cover the depth range from 80 to 114 meters below seafloor (mbsf), which—according to an astronomic time scale for Site 1062 (J. Grützner et al., unpubl. data)—is equivalent to an age interval of 655 to 1066 k.y. (Table T1). During sampling, preference was given to clay-rich sediments to avoid strong "dilution" of the sediment iron by biogenic hard parts. Moreover, care was taken that different sediment colors, ranging from reddish brown to dark olive green, were represented. Immediately after sampling (10 cm3 per sample), the sediment was double bagged in plastic and stored in a liquid nitrogen container that allowed shipping of the samples to our laboratory at temperatures below -40°C.
After 6 months of storage in the Bremen repository, cores were resampled at the same depth intervals. In order to avoid any additional alteration caused by transport, samples were packed in plastic bags and immediately cooled in liquid nitrogen. They remained in liquid nitrogen during transport and until measurement. The samples were investigated with respect to the distributions of the sediment iron among its two valence states Fe(II) and Fe(III), as well as the different iron minerals present, using Mössbauer spectroscopy. This element-specific method sensitively measures the hyperfine interactions between the iron nuclei and their electronic environment (Gütlich et al., 1978). Mössbauer spectra of bulk sediment samples are composite spectra, composed of subspectra that quantitatively represent the respective sedimentary iron species (Fig. F1). Whereas some of the subspectra are distinguishable, others overlap so much that they cannot be readily separated from each other. However, investigation of the samples at a variety of selected temperatures below room temperature, as well as the application of external magnetic fields during measurement, results in further distinction of individual subspectra.
A sequence of steps was followed systematically in order to achieve the maximum possible identification and quantification of different geochemical iron species. At room temperature, the respective fractions of Fe(II) in (1) silicate minerals, (2) siderite (FeCO3), and (3) vivianite [Fe3(PO4)2 · 8H2O], as well as (4) the overall sum of Fe(III) plus low-spin (sulfidic) Fe(II), can be determined (König and Hollatz, 1990). This differentiation is also possible at 77 K (-196°C), which helps prevent sample oxidation during measurement and was therefore the preferred temperature in the present study (Fig. F1A). To obtain further differentiation between individual chemical iron species in the sediment samples—that is, to discriminate between high-spin Fe(III) in silicate minerals, oxides, and oxyhydroxides as well as low-spin Fe(II) in sulfides—selected samples were also measured at 4.2 and 0.3 K (Fig. F1B), with and without external magnetic fields of 7 T (Tesla) and 1 T, respectively (A. Lougear et al., unpubl. data).
The error margin of the total amount of Fe(II)--without consideration of Fe(II) low-spin compounds such as pyrite--and correspondingly of Fe(III), consists of a statistical error (~0.5%) and a possible systematic error. The latter results from different Lamb-Mössbauer factors of Fe(II) and Fe(III) species. Recalibrating the Fe(II) amount using the Debye model (Gütlich et al., 1978), with Debye temperatures 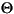 Fe(II) ~350 K and
Fe(II) ~350 K and 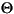 Fe(III) ~500 K (typical values for oxides, hydroxides, silicates, or carbonates; De Grave and Van Alboom, 1991), yields a systematic error of 1%.
Fe(III) ~500 K (typical values for oxides, hydroxides, silicates, or carbonates; De Grave and Van Alboom, 1991), yields a systematic error of 1%.