METHODOLOGY
Material from Leg 175 was recovered using advanced piston coring, and the retrieved cores were measured in meters below seafloor (mbsf) (Shipboard Scientific Party, 1998b). Upon return to shore, samples were freeze-dried overnight and weighed before subsampling for organic geochemistry analyses, magnetic characterization studies, and stable isotope analysis.
Material for further analysis was sampled at an interval of 0.5 m. Two sites were selected from the Congo Basin because of a paraconformity observed in the Hole 1076A shipboard results at 120 mbsf, indicating that material below this depth will be difficult to date and interpret. Hole 1076A was therefore sampled at 0.5-m intervals from 0 to 120 mbsf. Hole 1077A was also sampled at 0.5-m intervals from 70 to 160 mbsf. Material from Hole 1081A was sampled every 0.5 m from 0 to 100 mbsf, but with a particular focus on the interval between 40 and 100 mbsf. According to shipboard age-depth models, sampling every 0.5 m provides an age resolution of between 5,000 and 10,000 yr in all three holes.
Samples for stable isotopic analysis were wet sieved through a 63-µm sieve and separated into finer and coarser than 63-µm fractions before oven drying. Foraminifers were picked for isotope analysis from the coarse fraction. To minimize size effects, the samples were also dry sieved through a 250-µm sieve and individual foraminifer tests of each selected specimen were picked from the >250-µm fraction of each sample.
Four planktonic species, Globigerinoides ruber (white and pink), Globigerinoides sacculifer (with and without sac), Neogloboquadrina dutertrei, and Orbulina universa were selected for analyses. In addition, the benthic species Uvigerina peregrina and a mixed benthic assemblage consisting of mixed Cibicidoides taxa were also picked to generate two benthic isotope curves. For each planktonic species, between 10 and 30 individual tests were picked, and for each benthic group, up to 10 tests were picked.
Each foraminifer sample was reacted with phosphoric acid at 90°C in an online automated preparation system, and the resulting carbon dioxide was analyzed on a VG Prism ratio mass spectrometer for both oxygen and carbon isotope ( 18O and
18O and  13C) values. Corrections for the reaction between the acid and carbonate were applied to the results, and repeated analyses of standard material demonstrated that errors were <0.1
13C) values. Corrections for the reaction between the acid and carbonate were applied to the results, and repeated analyses of standard material demonstrated that errors were <0.1 for both
for both  18O and
18O and  13C. The results were then calibrated to the Peedee belemnite (PDB) scale, according to the NBS-19 standard.
13C. The results were then calibrated to the Peedee belemnite (PDB) scale, according to the NBS-19 standard.
Magnetic susceptibility was measured on discrete samples after return to shore. A small weighed amount of each sample (between 1 and 4 g) was packed tightly into a plastic bag to keep the material cohesive, and the bag was then placed in a small plastic vial. A blank vial was also prepared, containing only an empty plastic bag to allow measurements to be corrected for the containers. Measurements were made on the Kappabridge KLY-2 magnetic susceptibility system. Volume magnetic susceptibility measurements were recorded in 10-6 dimensionless SI units and corrected by subtracting the susceptibility of the container. The measurement was then converted to a mass-specific magnetic susceptibility (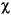 ) by dividing the volume susceptibility by the density (mass/volume) of each sample to obtain a measurement in 10-6 SI m3/kg. The volume of each sample was assumed to be 10 cm3, the volume approximated by the magnetic susceptibility system.
) by dividing the volume susceptibility by the density (mass/volume) of each sample to obtain a measurement in 10-6 SI m3/kg. The volume of each sample was assumed to be 10 cm3, the volume approximated by the magnetic susceptibility system.
Total Carbon and Total Organic Carbon
Total carbon (TC) concentrations (in weight percent) were obtained on subsamples of dry sediment of known weight (~0.5 g) using a Leco analyzer, which works by combusting the sample in an electric arc in the presence of oxygen and quantifies the carbon dioxide produced to give percentages of total carbon and total sulfur. A further subsample of sediment was fired at 500ºC for 24 hr to remove organic carbon, and the carbon content was then determined using the Leco analyzer to obtain total inorganic carbon (TIC) concentrations (in weight percent).
The total organic carbon (TOC) content (in weight percent) was determined from the difference between the total carbon and total inorganic carbon concentrations:
-
TOC = TC - TIC.
TOC concentration was converted to mass accumulation rate (MAR) to reduce the effects of dilution, using the equation from Emeis et al. (1995):
-
TOCMAR (mg/cm2/yr) = concentration (wt%)
× sedimentation rate (cm/yr) × density (g/cm3) × 10.
Linear sedimentation rates were calculated from the age-depth models, and gamma ray attenuation (GRA)-derived dry bulk density values were obtained from shipboard records (Shipboard Scientific Party, 1998b).
The organic material in the sediment was isolated using solvent extraction to investigate its organic components. The analysis was performed at the University of Newcastle, using the procedure based on the methodology of Rosell-Melé et al. (1995), detailed in Figure F3. Weighed subsamples of dried and homogenized sediments were processed partly with a robotic workstation. A known quantity of internal standard (n-C32 alkane [dotriacontane]) was added to the samples for later quantification, and the samples were extracted into dichloromethane (DCM) and methanol (3 mL:1 mL). After each extraction, the samples were centrifuged and decanted. The extraction process was conducted five times, until the extracted solvent became colorless. Combined extracts were dried under N2, leaving a green or yellow residue.
This residue was analyzed first for pigments on the spectrophotometer and then was derivatized with 50 µL of bis(trimethyl)silyltrifluoroacetamide (BST) and 50 µL of DCM before running on the gas chromatograph for lipid analysis.
Fractionation and Mass Spectrometry
Gas chromatography-mass spectrometry was used to obtain the Uk37´index from the C37 alkenones (Rosell-Melé et al., 1995). The Uk37´index is a measure of the degree of unsaturation of the C37 alkenone, which is made up of 37 carbon atoms (Brassell et al., 1986). This index considers the number of di- and tri-unsaturated compounds in the material such that
-
Uk37´= [C37:2]/(C37:2 + C37:3).
Based on comparisons of laboratory cultures and marine records (Prahl et al., 1988; Prahl and Wakeham, 1987), it has subsequently been demonstrated that this index increases linearly with SST in accordance with the following equation:
-
Uk37´= (0.033 × SST) + 0.043.
The C37:2 and C37:3 compounds were identified by running the extracted samples on a Carlo Erba gas chromatograph. Quantification was made by gas chromatography with split-splitless injection and flame ionization detection, using an HP 100% polymethylsiloxane column. Hydrogen (H2) was used as the carrier gas, with a head pressure of 100 kg/cm2. The oven temperature program was 45º to 205ºC at 20ºC/min, 205º to 305ºC at 10ºC/min, and 305ºC for 30 min. Data acquisition and integration were made with an Atlas data system. The alkenones were identified with reference to retention times based on authenticated standards, and the results were quantified using the internal standard. Peak areas were used to calculate the UK37´index, from which SSTs were then estimated using the standard equation of Prahl et al. (1988).
The chlorins and porphyrins in the solvent extracts of the sediments were identified using electronic spectrophotometry on a Philips PU8730 spectrophotometer. The residues of the extracted samples were diluted with between 5 and 15 mL of DCM, depending on their color. When the diluted sample yielded a pale yellow color, 3 mL of the liquid was poured into a cuvette and placed in a diode array spectrophotometer, which scans in the ultraviolet (UV)-visible wavelength interval (350-850 nm). The relative abundances of the chlorins and porphyrins were estimated by measuring the absorbance of the extracts in the regions close to the Soret band (S), which has highest extinction in the near-UV range (360-420 nm), and the Satellite band (I), close to 665 nm.
The relative absorbance value of the chlorinlike pigment (at 665 nm) was recalculated to give concentration values per gram as follows:
-
Concentration/g = (absorbance × dilution factor)/mass.
In addition, raw chlorin concentrations (in micrograms per gram) were converted to mass accumulation rates using the calculation from Emeis et al. (1995), according to the linear sedimentation rate calculated from the age-depth models and density measurements from GRA-derived dry bulk density values from shipboard records (Shipboard Scientific Party, 1998b), as follows:
-
Chlorin MAR (µg/cm2/yr) = concentration (µg/g)
× sedimentation rate (cm/yr) × density (g/cm3).
In the modern oceans, it has been established that phytoplankton productivity can be estimated from the concentrations of chlorophyll in seawater (Harris et al., 1996). Chlorophyll is the main pigment used in photosynthesis by higher plants and algae, which produce several pigmented organic compounds (Meyers, 1997). Chlorophyll, however, is not preserved in sediments, but chlorins and porphyrins, which are the transformation products of chlorophyll, are. Chlorins are the immediate diagenetic products of chlorophyll, whereas porphyrins result from long-term chlorophyll diagenesis (Rosell-Melé and Koç, 1997). Chlorin accumulation rates, calculated from density and sedimentation rates, have subsequently been shown to be a reliable and taxonomically independent indicator of paleoproductivity in areas of upwelling near Africa (Harris et al., 1996).
The ratio of the concentrations or absorbances at wavelengths of 410 and 665 nm can also be used to classify the pigments. By assuming that chlorinlike pigments have a wavelength close to 665 nm, whereas porphyrinlike pigments are represented by the 410-nm wavelength, it has been shown that chlorin-rich sediments have a 410/665 (porphyrin/chlorin) ratio between 1 and 5, whereas porphyrin-rich sediments have a higher value, between 5 and 10 (Rosell-Melé and Koç, 1997; Rosell-Melé et al., 1994).

 18O and
18O and  13C) values. Corrections for the reaction between the acid and carbonate were applied to the results, and repeated analyses of standard material demonstrated that errors were <0.1
13C) values. Corrections for the reaction between the acid and carbonate were applied to the results, and repeated analyses of standard material demonstrated that errors were <0.1 for both
for both  18O and
18O and  13C. The results were then calibrated to the Peedee belemnite (PDB) scale, according to the NBS-19 standard.
13C. The results were then calibrated to the Peedee belemnite (PDB) scale, according to the NBS-19 standard.
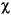 ) by dividing the volume susceptibility by the density (mass/volume) of each sample to obtain a measurement in 10-6 SI m3/kg. The volume of each sample was assumed to be 10 cm3, the volume approximated by the magnetic susceptibility system.
) by dividing the volume susceptibility by the density (mass/volume) of each sample to obtain a measurement in 10-6 SI m3/kg. The volume of each sample was assumed to be 10 cm3, the volume approximated by the magnetic susceptibility system.