INORGANIC GEOCHEMISTRY
Interstitial Water Chemistry
We squeezed 35 whole-round core samples for interstitial water at Site 1096. Two samples were taken from each of the first six cores in Hole 1096A, one sample from each of the next six cores, and one sample from every third core from Holes 1096B and 1096C (Table T25). We also obtained a single seawater sample from just above the mudline in Hole 1096B. Chloride concentrations in the upper 100 mbsf (Fig.
F31) exceed the modern bottom-water value by as much as 2.8% and could reflect the global increase in ocean salinity during the last glaciation (McDuff, 1985); however, the slightly increased variability of the chloride data within this interval may represent an artifact of sample processing or storage, thus limiting the strength of such an inference. Below 100 mbsf, chloride concentrations decrease steadily with depth by ~6%.
Organic Matter Degradation
The interstitial water chemistry at Site 1096 exhibits patterns similar to those observed at Site 1095, although generally with more pronounced concentration gradients (Fig.
F31). Decaying organic matter exerts a stronger influence at Site 1096, probably because the sediment has accumulated more rapidly (7 cm/k.y. in the upper 200 m) and contains slightly more organic carbon (0.1-0.4 wt%), particularly below 180 mbsf (see "Sedimentation
Rates" and "Organic
Geochemistry"). Dissolved manganese increases sharply with depth to a maximum concentration (196 µM) at 15 mbsf, reflecting dissolution of Mn oxides under suboxic conditions. Directly below this zone, dissolved sulfate and manganese decrease steadily with depth as a result of sulfate reduction and accompanying precipitation of sulfide minerals. Sulfate decreases to zero at 60 mbsf, where significant concentrations of methane and ethane first arise (see "Organic
Geochemistry"; Fig. F27), and manganese reaches a minimum concentration (9 µM) near 160
mbsf.
Other dissolved byproducts of organic matter decay, such as alkalinity, ammonium, and phosphate, all increase with depth in the upper sediment column (Fig. F31). Alkalinity reaches a sharp maximum (>8.0 mM) at 42 mbsf, a distinct minimum (4.0 mM) at 126 mbsf, and a broader maximum (>10.0 mM) between 200 and 400 mbsf, whereas ammonium increases steadily with depth to a maximum concentration (>2 mM) at 460 mbsf, then decreases slightly at greater depths. Unlike alkalinity and ammonium, dissolved phosphate increases to a maximum (>13.0 µM) at shallow depths, between 5 and 25 mbsf, then decreases somewhat erratically to 200 mbsf. At depths >200 mbsf, phosphate remains relatively constant (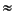 1.0 µM). Dissolved fluoride decreases sharply in the upper 50 mbsf and also remains constant (
1.0 µM). Dissolved fluoride decreases sharply in the upper 50 mbsf and also remains constant (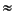 20 µM) at greater depths (Fig. F31). As at Site 1095, the phosphate and fluoride profiles suggest that apatite precipitates authigenically within the sediment, although probably in disseminated amounts too small to detect.
The constant phosphate and fluoride concentrations at depth could correspond to equilibrium values with respect to this mineral phase (Jahnke et al., 1983; Schuffert et al., 1994).
Note, however, that fluoride reaches a constant concentration at a shallower depth than phosphate, and the inferred equilibrium values differ slightly between Sites 1095 and 1096.
20 µM) at greater depths (Fig. F31). As at Site 1095, the phosphate and fluoride profiles suggest that apatite precipitates authigenically within the sediment, although probably in disseminated amounts too small to detect.
The constant phosphate and fluoride concentrations at depth could correspond to equilibrium values with respect to this mineral phase (Jahnke et al., 1983; Schuffert et al., 1994).
Note, however, that fluoride reaches a constant concentration at a shallower depth than phosphate, and the inferred equilibrium values differ slightly between Sites 1095 and 1096.
Dissolved iron concentrations remain near zero throughout most of the sediment column at Site 1096, but a small peak in dissolved iron occurs between 300 and 360 mbsf (Table T25), well below the zone where organic-matter diagenesis should dissolve the oxidized iron solid phases. This dissolved iron may relate, however, to the pyritized silt laminae that occur just below 360 m in Hole 1096C (intervals 178-1096C-19X-4, 110-115 cm, and 21X-2, 10-13 cm; see
"Lithostratigraphy"). Also, total sulfur concentrations increase at 357 mbsf and persist from 390 mbsf to the bottom of Hole 1096C (see "Organic
Geochemistry"; Table T23).
Silica, Carbonate, and Silicate Diagenesis
Other inorganic processes such as dissolution of biogenic silica and carbonate, reprecipitation of authigenic silica and carbonate phases, and diagenesis of clay and feldspar minerals probably influence the chemical composition of interstitial water at Site 1096. Dissolved silica increases considerably in the upper 5 to 50 mbsf (Fig. F31) and eventually attains maximum concentrations of ~1100 µM, essentially the solubility limit of opal-A (Kastner et al., 1977), at depths below 300 mbsf. Slightly lower dissolved silica concentrations occur between 100 and 150 mbsf, coincident with the interval of decreased alkalinity. We infer that biogenic opal dissolves principally between 0 and 50 mbsf, with substantial dissolution occurring in the uppermost meter of sediment because the first interstitial water sample contains much more dissolved silica (>500 µM) than the bottom-water sample (150 µM).
Overall, dissolved calcium increases slightly with depth in the upper 100 mbsf (Fig.
F31), but a distinct minimum in the calcium profile occurs at 50 mbsf, near the base of the sulfate reduction zone and coincident with a sharp reversal of the alkalinity gradient. Calcium remains fairly constant (~14 mM) between 100 and 400 mbsf, then increases below to a maximum value (25 mM) at the bottom of the hole. In contrast, dissolved magnesium decreases by >40% through the upper 150 mbsf (Fig.
F31) with a noticeable inflection at 50 mbsf, then remains constant (~30 mM) to 300 mbsf before decreasing further to a minimum value (14 mM) at the bottom of the hole. Similarly, dissolved potassium decreases by >60% through the upper 150 mbsf (Fig.
F31) with an inflection near 50 mbsf, but then remains constant (4 mM) to 500 mbsf before decreasing slightly to a minimum value (3 mM) at the bottom of the hole. Strontium remains constant at its seawater value (~90 µM) in the upper 30 mbsf (Fig.
F31), then increases steadily with depth to a constant value (~150 µM) below 300 mbsf.
From the profiles described above, we identify the base of the sulfate reduction zone as a particularly active horizon of mineral diagenesis at Site 1096. Dissolution of biogenic calcium carbonate and subsequent precipitation of authigenic carbonate phases could cause the observed release of strontium, the decrease in alkalinity, and the uptake of calcium and perhaps some magnesium. The occurrence near this horizon and at greater depths of narrow silt layers cemented partially by aragonite (see "Lithostratigraphy") provides direct evidence of authigenic carbonate precipitation.
Although the loss of dissolved sulfate enhances the possibility for replacement of calcite by dolomite (Baker and Kastner, 1981), we have not yet detected any direct evidence of dolomite formation at Site 1096. We speculate further that the silt layers could act as preferential pathways for upward diffusion of dissolved methane that could subsequently reoxidize to dissolved bicarbonate and conceivably trigger carbonate mineral growth at the base of the sulfate reduction zone, much as it does around methane seeps at the seafloor (Paull et al., 1992). If so, the carbonate-cemented silts should possess extremely light carbon-isotope signatures. By extrapolating the observed magnesium gradient beyond the maximum depth penetrated, we calculate that magnesium concentrations would decrease to zero at a depth of ~800 mbsf, well above the inferred basement depth of ~3500 mbsf (see "Seismic
Stratigraphy"); therefore, magnesium uptake probably results mostly from clay mineral diagenesis within the sediment column instead of from alteration of basaltic basement rocks (cf. Lawrence et al., 1975; Gieskes and Lawrence, 1976; Perry et al., 1976). In fact, clay mineral reactions probably exert the strongest influence on the observed calcium, magnesium, potassium, and perhaps strontium profiles.
X-Ray Diffraction Mineralogy
A total of 28 samples were analyzed by X-ray diffraction for clay mineralogy at Site 1096. All but one of these were also analyzed for their bulk mineralogy. In three cores, two samples were taken where alternating colors were interpreted as possible barren glacial and biogenic interglacial intervals. Most unpaired samples were taken from presumed glacial intervals. Bulk and clay mineralogies at Site 1096, which were similar to those found at Site 1095, consist primarily of quartz, feldspar, and a mixture of clay minerals, including chlorite, illite, and a mixed-layer clay, most likely mixed smectite-illite of varying proportions. Traces of amphibole were also detected in all samples.
The relative abundances of quartz and feldspar, as measured by the ratio of their principal diffraction peaks, remain nearly constant among all samples, but clay mineral abundances vary significantly. In all but a few bulk mineral samples, the 3.19-Å plagioclase diffraction peak ranges between 30% and 45% of the height of the 3.34-Å quartz peak (Table T26). Considerable variability among the clays, however, is demonstrated by the widely varying ratios between their diffraction intensities (Table T27; Fig. F32). This is particularly evident in the relative intensity of the mixed-layer peak, which varies among samples by an order of magnitude relative to chlorite. In general, the greatest abundances of chlorite and mixed-layer clay relative to illite occur below 200 mbsf. As at Site 1095, however, the greatest variability among clays occurs between alternating sediment types instead of as a function of age or burial depth.
X-Ray Fluorescence and Trace-Element Chemistry
Trace-element concentrations were measured by X-ray fluorescence on splits of the 27 bulk-sediment samples analyzed by X-ray diffraction. These results (Table T28) are similar to those obtained from Site 1095. For most elements analyzed, peak concentrations occur in the uppermost 150 mbsf, typically between 40 and 150 mbsf, and then decrease by ~30% with greater depth. For example, Rb concentrations increase from 90 to 120 ppm between 20 and 60 mbsf and then decrease to ~75 ppm at 320 mbsf, a value typical of the lower 250 m in Hole 1096C. These trends imply that similar factors influenced the bulk-sediment chemistry at Sites 1095 and 1096. At both sites, the trend toward lower concentrations of lithophile trace elements (e.g., Rb, Cr) occurs in sediments of similar age ~1.5 Ma (see "Sedimentation
Rates"; also see "Sedimentation Rates"
in the "Site 1095" chapter). This change in composition correlates poorly with interstitial water chemistry profiles. For example, much sharper interstitial concentration gradients occur at Site 1096 than at Site 1095, reflecting the shallower occurrence of sulfate reduction and other redox-related reactions. In contrast, sharper bulk-sediment chemical gradients are found at Site 1095. Based on these observations, we conclude that the sedimentary trace-element gradients at these two sites result primarily from changes in sediment source (i.e., provenance) rather than from reactions with interstitial water. The peak concentration of Ba (1250 ppm) in the shallowest interglacial sample implies high surface-water productivity during deposition of this interval (Dymond et al., 1992), whereas the absence of high barium concentrations in older interglacial intervals may reflect dissolution of barium under anoxic conditions (Brumsack and Gieskes, 1983; von Breymann et al., 1990).

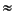 1.0 µM). Dissolved fluoride decreases sharply in the upper 50 mbsf and also remains constant (
1.0 µM). Dissolved fluoride decreases sharply in the upper 50 mbsf and also remains constant (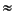 20 µM) at greater depths (Fig. F31). As at Site 1095, the phosphate and fluoride profiles suggest that apatite precipitates authigenically within the sediment, although probably in disseminated amounts too small to detect.
The constant phosphate and fluoride concentrations at depth could correspond to equilibrium values with respect to this mineral phase (Jahnke et al., 1983; Schuffert et al., 1994).
Note, however, that fluoride reaches a constant concentration at a shallower depth than phosphate, and the inferred equilibrium values differ slightly between Sites 1095 and 1096.
20 µM) at greater depths (Fig. F31). As at Site 1095, the phosphate and fluoride profiles suggest that apatite precipitates authigenically within the sediment, although probably in disseminated amounts too small to detect.
The constant phosphate and fluoride concentrations at depth could correspond to equilibrium values with respect to this mineral phase (Jahnke et al., 1983; Schuffert et al., 1994).
Note, however, that fluoride reaches a constant concentration at a shallower depth than phosphate, and the inferred equilibrium values differ slightly between Sites 1095 and 1096.