-
(CH2O)106(NH3)16(H3PO4) + 53 SO42- + 14 H+
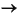 106 HCO3-+ 16 NH4+ + HPO42- + 53 H2S,
106 HCO3-+ 16 NH4+ + HPO42- + 53 H2S,
From the downward variation of dissolved sulfate concentrations in the interstitial waters (Fig. F2; Table T1), it is evident that all sites are characterized by more or less intense bacterial sulfate reduction throughout the sediment column. Sulfate reduction associated with the degradation of organic matter leads to the liberation of carbon dioxide, ammonium, and hydrogen sulfide (Froelich et al., 1979; Gieskes, 1981) according to the overall reaction
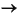 106 HCO3-+ 16 NH4+ + HPO42- + 53 H2S,
106 HCO3-+ 16 NH4+ + HPO42- + 53 H2S,
in general agreement with the observed downcore variations in sulfate, alkalinity, and ammonium (Fig. F3) (Carter, McCave, Richter, Carter, et al., 1999). Besides mineralization of organic matter, alkalinity data were additionally influenced by the carbonate diagenesis and sorption on clay minerals, respectively. Dissolved sulfate was completely exhausted within the first 20 m at Sites 1119 and 1122, indicating that rates of sulfate reduction exceed the supply rate of sulfate from the sediment/water interface. At Site 1123, most of the sulfate reduction seems to occur within the first 150 m of the sediment column. Low net sulfate reduction rates are found at Site 1120, 1121, and 1124.
Microbial reduction of dissolved sulfate causes a kinetic isotope effect. An enrichment of the lighter sulfur isotope 32S in the formed hydrogen sulfide is the result (Kaplan and Rittenberg, 1964; Chambers and Trudinger, 1979), leading to a corresponding accumulation of the heavy isotope (34S) in the residual sulfate (Hartmann and Nielsen, 1969; J°rgensen, 1979). Therefore, even at Site 1120 where the pore water sulfate concentrations only decrease slightly with depth (Fig. F2), microbial activities consuming sulfate as the electron acceptor are recorded by the sulfur isotopic composition of residual sulfate.
Reduction of dissolved sulfate in the sediment is coupled to the availability of metabolizable organic matter or hydrocarbons (mainly CH4) (Berner 1980; Borowski et al. 1996; Hoehler et al., 1994; Boetius et al. 2000). In agreement with a controlling function of organic matter contents on the microbial activity, sulfate gradients are positively correlated to the bulk sedimentation rates (Carter, McCave, Richter, Carter, et al., 1999), which indicates higher preservation of metabolizable organic matter with an increasing sedimentation rate (Berner, 1980). The highest sedimentation rate is found in the upper parts of Sites 1119 and 1122 (Fig. F4), corresponding to a fast exhaustion of dissolved sulfate (Fig. F2). According to shipboard measurements, methane increased only where sulfate was completely depleted from the pore waters at Sites 1119 and 1122 (Carter, McCave, Richter, Carter, et al., 1999). In the zones of complete sulfate exhaustion at these sites, the dissolution of biogenic barite can be expected according to the concept of the development of a diagenetic "barite fronts" (Brumsack et al., 1992; Torres et al., 1996). Following this model, diagenetic baryte formation may be initiated at the meeting points of upward-diffusing barium with downward-diffusing sulfate. Based on the isotopic composition of dissolved sulfate (~+60 vs. V-CDT), this diagenetic barite should be highly enriched in 34S with respect to modern biogenic baryte (Paytan et al., 1998) and Pacific Ocean water sulfate (Longinelli, 1989; B÷ttcher et al., 2000).
vs. V-CDT), this diagenetic barite should be highly enriched in 34S with respect to modern biogenic baryte (Paytan et al., 1998) and Pacific Ocean water sulfate (Longinelli, 1989; B÷ttcher et al., 2000).
The sulfate profiles at Sites 1119 and 1123 show a convex-up curvature (Fig. F2) which, together with the downward variations in alkalinity and dissolved ammonium (Carter, McCave, Richter, Carter, et al., 1999), indicate that sulfate reduction seems to be caused by in situ microbial degradation of organic matter and that upward diffusion of methane here plays no role in the upper part of the sedimentary column (Borowski et al., 1996).
Although sulfate reduction should be less significant in the deep sea when compared to continental shelf sediments (J°rgensen, 1982; Canfield, 1991), the highest net sulfate reduction was found at Site 1122 (4432 m water depth). However, below ~200 meters below seafloor (mbsf), sulfate started to increase again with depth and reached an apparent steady-state value below ~500 mbsf. The sulfur isotopic composition in the deep sediment layers decreased over this interval, but both parameters did not reach bottom-water conditions. This indicates that the deep pore waters had been influenced by microbial sulfate reduction. The dramatic change in the pore water composition in the deep sediment column corresponds to a change in the sedimentation rate from >40 cm/k.y. down to a rate of ~5 cm/k.y. (Fig. F3), which is substantiated by lithostratigraphic units and sedimentological properties. The upper 300 m of sediment consists of rhythmic upper Pleistocene sand turbidites and below 500 mbsf of upper Miocene current-influenced sands and muds. At ~260 mbsf, a high abundance of pyrite was observed at the base of the mud-wave sequence (Carter, McCave, Richter, Carter, et al., 1999), probably a result of enhanced microbial activity and/or some sorting phenomena during the turbidite settling processes. An unconformity exists at ~470-500 mbsf. The control of sulfate reduction by turbidites as carriers of reactive organic matter into the deep sea has been observed earlier in sediments of the deep Arabian Sea (~4040 m water depth) (B÷ttcher et al., 2000). Turbiditic sediments with enhanced organic carbon contents stimulated both microbial net (B÷ttcher et al., 2000) and gross (Boetius et al., 2000) sulfate reduction even in near-surface sediments.
TRIS contents measured at Site 1119 (Table T2) fit into the range of shipboard measurements of total sulfur; however, the TRIS data show no close covariaton with total organic carbon contents (Fig. F5). Under steady-state closed-system conditions, continuous increase of pyrite with depth until exhaustion of dissolved sulfate would be expected (Hartmann and Nielsen, 1969). Therefore, the strong fluctuations of TRIS contents in the sediment profile of Site 1119 probably indicate a dominant formation of pyrite close to the sediment/water interface (Berner, 1980) with some minor contributions from the zone of active net sulfate reduction.
In conclusion, sulfur isotope measurements on the dissolved sulfate of interstitial waters from the southwest Pacific clearly indicate the activity of sulfate reducing bacteria. Prime control for this process would be the delivery of metabolizable organic matter. This seems to be partly provided via turbidite sedimentation, as suggested by a coupling of high sedimentation rate and high sulfate turnover.