- Mg2+ +
2CaCO3
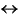 CaMg(CO3)2 + Ca2+
CaMg(CO3)2 + Ca2+
Concentrations of volatile hydrocarbon gases were measured from every core using the standard ODP headspace sampling technique and gas chromatographic analysis. Methane only occurred in very minor concentrations (2 ppmv); near the surface, methane content increases slightly to 11 ppmv (Table T8).
The low gas content at Site 1192 is likely a function of three characteristics of the sediment. First, the sediments contain very little organic matter as a source of natural gas. Second, pore water profiles show that appreciable SO42- exists to the bottom of the hole; thus, sulfate reduction is likely to be limiting methanogenesis in this interval. Third, the organic matter that does exist here is immature relative to hydrocarbon expulsion and petroleum generation, so no thermogenic component to the gas fraction is expected.
Pore water samples were taken approximately every 10 m in Hole 1192A, which reached a total depth of 240 mbsf. After having taken one core close to the mudline, Hole 1192B was washed to 179.9 mbsf and was then cored to 345.0 mbsf. Additional pore water samples were taken at 10-m intervals in Hole 1192B. Results from both holes are discussed together.
The concentrations of dissolved constituents at Site 1192 (Table T9; Fig. F17) are relatively constant with depth compared to changes observed at many other ODP sites. Dissolved chloride decreases from a value at the sediment surface of 570 to ~562 mM at 140 mbsf and increases again to 570 mM at 340 mbsf near the bottom of Hole 1192B (Fig. F17A). Titrated alkalinity increases very slightly in the upper 40 m at this site, from 1.7 to 2.4 meq/L, and thereafter varies unsystematically between 1.8 and 3.2 meq/L (Fig. F17B).
Dissolved potassium concentrations decrease downhole from 12 mM in the shallowest samples to ~6.5 mM in the deepest samples (Fig. F17C). Such a decrease has been observed in many Deep Sea Drilling Project (DSDP)/ODP sites and is usually attributed to clay mineral diagenesis. The concentration of lithium in the pore waters increases from ~30 µM near the sediment surface to 150 µM at the bottom of Hole 1192B.
Magnesium concentrations decrease downhole from near seawater concentrations (55 mM) in surface sediments to ~38 mM toward the bottom of Hole 1192B (Fig. F17E). Calcium concentrations increase downhole from 12.1 mM at 5.9 mbsf to ~18 mM at 342 mbsf (Fig. F17D). It is not clear whether the decrease in Mg2+ is caused by carbonate precipitation or is simply a diffusion gradient between seawater and basement rocks underlying the sediments. The latter mechanism has often been used to explain similar trends in pelagic sediments underlain by oceanic crust, which acts as a sink for magnesium and a source for calcium because of low-temperature basalt alteration. Basement underneath the Marion Plateau drilled at Site 1194 consists of generally basaltic volcanics and volcaniclastic sediments. More mafic components are also present. Alternatively, dolomite precipitation via the reaction
could remove magnesium and simultaneously input calcium to the pore waters. This mechanism is supported by the presence of minor amounts of fine-grained dolomite through much of the sediment column (see "Lithostratigraphy and Sedimentology"). Carbonate precipitation would also explain the low alkalinity values. Even the modest amount of sulfate reduction at Site 1192 would normally be expected to be associated with increasing alkalinity in a 1:2 ratio. Dolomitization by the above reaction should produce an approximately linear trend in Ca2+ against Mg2+, with a slope of -1. Such a trend is not seen (Fig. F18). Mg2+ is highly correlated to SO42-, however (Fig. F19), which suggests dolomite formation might be proceeding through the less commonly observed reaction
with the bicarbonate sourced from sulfate reduction. This reaction results in a 2-mM reduction in alkalinity per mole of Mg2+ consumed; 1 mole of SO42- reduced to sulfide should produce 2 moles of alkalinity. It follows that the overall Mg2+:SO42- ratio for dolomitization should be 1:1, approximately what is observed in the pore waters of Site 1192. Thus, Mg2+ is likely removed from the pore waters by dolomite formation, whereas Ca2+ is added by alteration of basement rocks. These two independent processes explain why there is no correlation between pore water Ca2+ and Mg2+ concentrations.
As a result of carbonate recrystallization, strontium concentrations increase steadily downhole through Site 1192 sediments (Fig. F17F). At ~250 mbsf, a value of ~800 µM is reached. The high strontium concentration and low amount of sulfate reduction result in celestite precipitation in the deeper parts of the sediment section at Site 1192.
In response to a moderate rate of bacterial sulfate reduction, dissolved sulfate (Fig. F17G) decreases from 30.4 mM in the shallowest sample at 5.9 mbsf to ~18 mM at the bottom of Hole 1192A. A further decrease to 15 mM is seen through the section drilled in Hole 1192B. The oxidation of sedimentary organic matter associated with sulfate reduction results in a steady increase in ammonium concentrations (Fig. F17H) throughout Site 1192. Values near the sediment surface are 24 µM and are ~1000 µM at the bottom of Hole 1192B. Sulfate and ammonium are highly negatively correlated (r2 = 0.96).
Manganese and iron
concentrations were also measured in all Site 1192 samples. Manganese was below
detection limits (i.e., were within the 1-![]() error of 0) in all samples. Iron values were also either very low or within a
measurement error of 0 (Fig. F17I).
In all likelihood, the low organic carbon concentration in the sediments results
in limited reduction of iron and manganese oxides and subsequent input to the
pore fluids. The small amount of iron or manganese that is released to the pore
water is probably rapidly precipitated as sulfide minerals. This conclusion is
supported by the presence of Fe sulfide minerals through much of the sedimentary
section.
error of 0) in all samples. Iron values were also either very low or within a
measurement error of 0 (Fig. F17I).
In all likelihood, the low organic carbon concentration in the sediments results
in limited reduction of iron and manganese oxides and subsequent input to the
pore fluids. The small amount of iron or manganese that is released to the pore
water is probably rapidly precipitated as sulfide minerals. This conclusion is
supported by the presence of Fe sulfide minerals through much of the sedimentary
section.
Calcium carbonate (CaCO3) content in samples from Site 1192 range from ~66 to 94 wt% (Fig. F20; Table T10). Calcium carbonate content exhibits an overall decrease from values of ~91 wt% at ~2 mbsf to ~73 wt% at ~51 mbsf. From ~51 to 99 mbsf, an increasing trend in weight percent CaCO3 was measured, with values ranging from ~74 to 82 wt%. Between ~99 and 263 mbsf, calcium carbonate content is consistently high at ~80-92 wt%. Sediments from ~263 to ~343 mbsf display the entire range in CaCO3 contents observed at Site 1192, including two excursions with low weight percent CaCO3 values (<70 wt%) at 262.86 and 327.85 mbsf.
The total organic carbon content for all intervals at Site 1192 is <0.3 wt%. Note that weight percent total organic carbon (TOC) values determined using Rock-Eval pyrolysis and CNS analysis provide similar values (Fig. F20; Tables T10, T11). Hydrogen index (HI) values from Rock-Eval pyrolysis range from 83 to 366 mg HC/g TOC at Site 1192 (Fig. F20; Table T11). The lowest values were observed in horizons between 0-6, 55-105, 157, 239-245, and 328-329 mbsf. The highest HI value occurs in sediments at ~137.25 mbsf. Oxygen index (OI) values vary between 411 and 3785 mg CO2/g TOC (Table T11). Tmax values obtained from Rock-Eval pyrolysis range from 364° to 435°C (Table T11), although the most reliable Tmax values cluster between 400° and 420°C.
The low percent TOC values obtained from Rock-Eval pyrolysis of the sediment at Site 1192 limit the reliability of some of the HI and OI values. However, duplicate and triplicate analyses were performed on many of the sediments to validate the results. The high OI values measured are attributed to the thermal degradation of calcium carbonate during pyrolysis and are not considered in this interpretation.
Total sulfur content in Site 1192 sediments is generally low, ranging from 0 to >0.4 wt% (Fig. F20; Table T10). Intervals of relative sulfur enrichment are visible at ~64-113, 151-165, 241, and 310-330 mbsf.
The high calcium carbonate content of sediments at Site 1192 mainly reflects dominance of foraminifers in the sediments (see "Biostratigraphy and Paleoenvironments" and "Lithostratigraphy and Sedimentology"). The downhole increase in calcium carbonate content below ~99 mbsf corresponds to an increase in color reflectance and benthic constituents preserved in the sediment. These changes are suggestive of increased input of detritus from the adjacent carbonate platforms to the sediments of Site 1192. From ~99 to 263 mbsf, calcium carbonate contents display reduced variability, corresponding to an interval of sediment dominated by planktonic foraminifers. Interestingly, two intervals within this zone exhibit calcium carbonate contents ranging from ~84 to >90 wt% and are separated by an interval of sediment containing 80 to 85 wt% CaCO3. These two intervals (~100-160 and ~200-250 mbsf) correspond to units containing firmgrounds, suggesting that episodes of seafloor sediment starvation affected calcium carbonate preservation. The zone of widely ranging calcium carbonate contents from ~263 to ~343 mbsf corresponds to observations of fine-grained carbonate sediments of unknown origin mostly barren of planktonic foraminifers. This zone from ~263 to ~343 mbsf also corresponds to lithologic Units III and IV, where the content of glauconite and phosphate grains is notable and dolomite rhombs and quartz were observed.
The low organic carbon contents preserved in the sediments at Site 1192 is suggestive of a well-mixed water column and/or relatively oxic seafloor conditions during deposition. The Tmax values obtained from Rock-Eval pyrolysis are indicative of immature organic matter; therefore, we are confident that our analyses of organic matter type (discussed below) record a primary signal. The anomalously low Tmax values just beneath the seafloor are likely attributable to the presence of relatively "fresh" Type I organic matter, which can generate erroneously low Tmax values.
The type of organic matter encountered provides further insight into depositional processes at Site 1192. Both C/N ratios and HI values are indicative of mostly marine to oxidized marine organic matter preservation. However, at least four intervals were observed that display HI values (<150) characteristic of terrigenous or oxidized marine organic matter with relative enrichments in total sulfur content. These horizons either correspond to decreases in carbonate content or the existence of firmgrounds (see "Lithostratigraphy and Sedimentology"), suggesting that episodes of enhanced terrigenous or oxidized marine organic matter input/preservation to the seafloor occurred during those times when carbonate deposition waned. Increased sulfur content may be indicative of iron limitation during pyrite formation in the intervening sediments, as the total organic carbon content is similar throughout the sediment column. Therefore, we might expect increased clay content (as a source of reduced iron) to be associated with these relatively elevated terrigenous organic matter and sulfur intervals.
Of further interest are four relative peaks in C/S values (>2) at Site 1192, which may record episodes of brackish pore water in the sediments. Admittedly, the use of the C/S method may be limited in high carbonate content sediments (Berner and Raiswell, 1984). However, in each case, the C/S peaks correspond to relatively lower carbonate contents and exist ~20 m beneath the intervals of elevated terrigenous organic matter and sulfur content. Therefore, episodes of terrigenous organic matter input to the seafloor may have been accompanied by the presence of brackish pore waters in a biologically mediated diagenetic depth zonation in the underlying sediments.
Within a broader sequence stratigraphic perspective, some of the geochemically defined intervals correspond to major seismic sequence boundaries (see "Seismic Stratigraphy"). The most instructive is the recognition of the boundary between seismic sequences C and B at ~240 mbsf. This surface closely corresponds, within the range of seismic resolution and depth uncertainties, to a geochemically defined boundary between ~234 and 260 mbsf (i.e., low HI and high C/N values [terrigenous or Type III organic matter] overlain by a relatively high HI value [marine organic matter]). These observations may be explained by terrigenous organic matter deposition within a relative lowstand setting, whereas marine organic matter was deposited during subsequent marine flooding. Furthermore, the lowest carbonate content at Site 1192 was measured at ~263 mbsf, suggesting that a break in production on the carbonate platform, likely by an exposure, was followed by more terrigenous organic matter inputs and/or organic matter oxidation.
Between ~142 and 157 mbsf, the entire range in HI values at Site 1192 was observed; therefore, a similar sequence stratigraphic model for organic matter deposition/preservation may be invoked. Here, however, the seismic sequence boundary pick at ~120 mbsf does not correspond to the geochemically defined surface, suggesting that higher-frequency geochemical cycles may represent parasequence-scale deposition.