|
Biomarker class
components (code) |
Range
|
Relative abundance
|
Putative biological source(s)/process
of formation |
References
|
||||
|---|---|---|---|---|---|---|---|---|
|
8R-1,
47 cm |
8R-1,
63 cm |
8R-1,
96 cm |
15R-1,
9 cm |
19R-1, 112 cm
|
||||
| n-Alkyl compounds: |
|
|
|
|
|
|
|
|
| Lower alkanes with OEP | C13-C19 | +++ | +++ | ++ | ++ | +++ | Algae, bacteria, cyanobacteria | Han et al., 1968; Gelpi et al., 1970; Brassell et al., 1978 |
| Higher alkanes with OEP | C23-C33 | ++ | ++ | + | + | ND | Vascular plants | Eglinton and Hamilton, 1963; Simoneit, 1978 |
| Branched compounds: |
|
|
|
|
|
|
|
|
| Monomethylalkanes (MA) | C14-C17 | + | + | ++ | + | ++ | Bacteria, cyanobacteria | Shiea et al., 1990; Dachs et al., 1998; Köster et al., 1999 |
| Acyclic isoprenoids: |
|
|
|
|
|
|
|
|
| TMTD, TMPD | C16, C18 | +++ | +++ | ++ | ++ | ++ | Photoautotrophs | Didyk et al., 1978; Volkman and Maxwell, 1986 |
| Pristane (PR) | C19 | ++ | + | + | + | ++ | Photoautotrophs | Didyk et al., 1978; Goossens et al., 1984 |
| Phytane (PH) | C20 | +++ | ++ | ++ | + | + | Photoautotrophs, methanogens | Didyk et al., 1978; Brassell et al., 1981 |
| Hexamethyltetracosane (HMTC) | C20 | ++ | ND | ++ | ND | ND | Methanogenic bacteria |
|
| Steroids: |
|
|
|
|
|
|
|
|
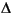 4- and 4- and 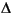 5-sterenes 5-sterenes |
C27-C29 | +++ | +++ | +++ | +++ | +++ | Eukaryotes/sterol dehydration | Mackenzie et al., 1982; Peakman and Maxwell, 1988 |
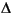 4,22- and 4,22- and 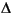 5,22-steradienes 5,22-steradienes |
C29 | ++ | ++ | ++ | +++ | + | Eukaryotes/sterol dehydration | Rullkötter et al., 1984; Farrimond et al., 1986b |
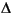 3,5- steradienes 3,5- steradienes |
C27-C29 | ND | + | ND | ND | ND | Eukaryotes/sterene rearrangement | Brassell et al., 1984; Peakman and Maxwell, 1988 |
| A-ring monoaromatic | C27-C29 | + | ++ | + | ++ | + | Eukaryotes/sterol aromatization | Hussler et al., 1981; Brassell et al., 1984 |
| B-ring monoaromatic anthra- | C27-C29 | + | + | + | ND | ND | Eukaryotes/sterol aromatization | Hussler and Albrecht, 1983; Rullkötter and Welte, 1983 |
| Triterpenoids: |
|
|
|
|
|
|
|
|
17 (H),21 (H),21 (H)-hopanes (H)-hopanes |
C27, C29-C31 | +++ | +++ | +++ | +++ | +++ | Prokaryotes | Ourisson et al., 1979, 1987; Rohmer et al., 1992 |
17 (H),21 (H),21 (H)-hopanes (H)-hopanes |
C27, C29-C31 | ++ | ++ | ++ | + | + | Prokaryotes | Ourisson et al., 1979, 1987; Rohmer et al., 1992 |
17 (H),21 (H),21 (H)-methylhopanes (H)-methylhopanes |
C30-C33 | ++ | ND | ++ | ND | ND | Cyanobacteria, methylotrophs | Ourisson et al., 1987; Summons and Jahnke, 1990 |
| Hopanes > C32 | C33 | +++ | + | + | ND | ND | Bacteria, methylotrophs | Ourisson et al., 1979, 1987; Rohmer et al., 1992 |
| Hop-17(21)-enes | C27, C29-C31 | +++ | ++ | ++ | +++ | ++ | Bacteria, anaerobic phototrophs | Brassell and Eglinton, 1983; Rohmer et al., 1984 |
| Methylhopenes | C27 | ++ | ND | ++ | ND | ND | Cyanobacteria, methylotrophs | Ourisson et al., 1987; Summons and Jahnke, 1990 |
| Aromatic hopanoid | C27 | ND | + | + | ND | ND | bacteria/aromatization | Greiner et al., 1976, 1977 |
| Neohop-13(18)-enes | C27, C29, C3 | ++ | ++ | ++ | ND | + | Bacteria, anaerobic phototrophs | Brassell and Eglinton, 1983; Howard et al., 1984 |
| Fernenes | C30 | ++ | ++ | + | +++ | ++ | Bacteria, anaerobic phototrophs | Brassell and Eglinton, 1983; Howard et al., 1984 |
| triterpene (dammaradiene?)C30 |
|
ND | +++ | ND | ND | ++ | Bacteria? | Brassell, 1984; Rullkötter et al., 1984 |
| Ketones and ethers: |
|
|
|
|
|
|
|
|
| Alkan-2-ones | C13-C23 | + | + | + | + | + | Algae, bacteria/oxidation | Brassell et al., 1980 |
| Alkadienones | C37-C39 | ND | ND | ++ | ND | ND | Haptophyte algae | Brassell et al., 1986; Farrimond et al., 1986b; Marlowe et al., 1990 |
| TMTD-2-one | C18 | + | ++ | + | + | + | Photoautotrophs; oxidation | Simoneit, 1973 |
5 (H)- and 5 (H)- and 5 (H)-stanones (H)-stanones |
C27-C29 | +++ | +++ | +++ | +++ | +++ | Eukaryotes/sterol oxidation | Gagosian and Smith, 1979; Robinson et al., 1984 |
| 4-methylstanones | C28-C30 | ++ | + | ++ | ++ | +++ | Dinoflagellates | Gagosian and Smith, 1979; Robinson et al., 1984 |
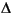 4-stenones 4-stenones |
C27-C29 | ++ | ++ | +++ | ++ | ++ | Eukaryotes/sterol oxidation | Edmunds et al., 1980; Brassell et al., 1987 |
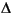 4,22-stenones 4,22-stenones |
C27-C29 | ++ | +++ | ++. | ++ | ++ | Eukaryotes/sterol oxidation | Edmunds et al., 1980; Brassell et al., 1987 |
4-methyl-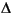 22-sterones 22-sterones |
C28-C31 | + | + | + | + | ++ | Dinoflagellates | Gagosian and Smith, 1979; Robinson et al., 1984 |
| Hopanones | C27, C29-C31 | ++ | ++ | NA | ++ | ++ | Bacteria, algae/predation, oxidation | Dastillung et al., 1980 |
| Methylhopanones | C30-C33 | ++ | + | ++ | ND | ND | Bacteria, algae/predation, oxidation | Dastillung et al., 1980 |
| Methylmethylhopanyl ether | C32 | ++ | + | +++ | ND | ND | Bacteria | Dastillung et al., 1980; Rohmer et al., 1992 |
| Other biomarkers: |
|
|
|
|
|
|
|
|
 -, -,  -, -,  -, -,  -tocopherols -tocopherols |
C27-C29 | ++ | ++ | ++ | + | + | Photoautotrophs | Brassell et al., 1983; Goossens et al., 1984 |
| Sterol ethers | C27-C29, C9 | ND | ND | ND | +++ | ND | Unknown | Boon and de Leeuw, 1979; Brassell et al., 1980 |
Notes: TMTD = 2,6,10-trimethyltridecane, TMPD = 2,6,10-trimethylpentadecane. +, ++, +++ = approximate relative abundances corresponding to major, minor, and trace amounts. ND = not detected. NA = not analyzed. OEP = odd/even preference.
![]()