BIOSTRATIGRAPHY
Pleistocene-middle Eocene strata were recovered at Site 1218 and permit the development of an integrated calcareous and siliceous biozonation (Fig. F7). All microfossil groups, with the exception of radiolarians, have sporadic occurrences and often poor preservation in the upper Neogene sequence but become better preserved in the lower Miocene-Oligocene interval. Radiolarians are consistently present in the recovered material, but their abundance and preservation are variable in the younger parts of the Neogene. The presence of expanded, well-preserved radiolarian fauna throughout the Miocene-Eocene section will permit the detailed calibration of the tropical radiolarian zonation to magnetostratigraphy and calcareous microfossil stratigraphy. The O/M boundary is well constrained by radiolarian and foraminiferal datum events, and the presence of the nannofossil S. delphix provides an additional reliable marker close to the boundary. At Site 1218, we recovered a complete succession of Oligocene and upper Eocene biozones, including a record of the E/O boundary interval with a well-defined radiolarian and calcareous nannofossil biostratigraphy. Calcareous nannofossils generally suffer from some dissolution but were sufficiently well preserved to construct a detailed biozonation in the Eocene, whereas planktonic foraminifers have been completely removed from upper Eocene sediments and are only represented by dissolution-resistant taxa in the middle Eocene. Benthic foraminifers are present in most samples of lower Miocene-middle Eocene sediments and represent a cosmopolitan bathyal to abyssal fauna.
Calcareous nannofossils are present at varying concentrations and states of preservation from the uppermost middle Miocene Zone NN8 (CN6) in Core 199-1218A-4H to the basal part of the middle Eocene Zone NP16 (CP14a) in Core 30X. Nannofossils are lacking from the seafloor to Sample 199-1218A-4H-6, 50 cm, because of calcite dissolution. Below this barren interval, assemblages are consistently affected by calcite dissolution, albeit less so in the lower Miocene and Oligocene carbonate-rich assemblages than in the Eocene assemblages. Preservation is generally poor in the condensed middle Miocene sediments, except for discoasters. Depth positions and age estimates of key biostratigraphic marker events are shown in Table T3.
Miocene
The uppermost carbonate-bearing sediments in interval 199-1218A-4H-6, 85 cm, contain a low-diversity assemblage consisting chiefly of Discoaster spp., Catinaster spp., and Triquetrorhabdulus rugosus, suggesting that Site 1218 last moved below the CCD during Zone NN8 (CN6) time. Discoaster kugleri is present in Sample 199-1218A-4H-CC, placing this sample in Zone NN7 (CN5b), which corresponds to Sample 199-1218B-5H-2, 56 cm.
Sample 199-l218B-5H-CC held the highest occurrence of both Cyclicargolithus floridanus and Coronocyclus nitescens and the lowest occurrence of T. rugosus. Rare aberrant forms with Triquetrorhabdulus affinity are also present in Sample 199-1218B-5H-CC, presumably reflecting the evolutionary transition creating the T. rugosus lineage from Orthorhabdus serratus. This core catcher sample also contains a well-preserved discoaster assemblage lacking calcite overgrowth with abundant Discoaster exilis and other species belonging to the Discoaster deflandrei, D. exilis, Discoaster variabilis group and beautifully developed Discoaster signus, having a range in lower Zone NN5 (CN4) and upper NN4 (CN4-CN3 transition).
Section 199-1218B-6H-1 has a discoaster assemblage with abundant D. deflandrei. This marked change in proportion among discoasters indicates a position below the CN4/CN3 boundary when using Bukry's (1973) top acme D. deflandrei concept as boundary definition. The corresponding change in Hole 1218A is observed between Samples 199-1218A-5H-6, 15 cm, and 5H-CC. In this latter sample, only rare specimens of Sphenolithus heteromorphus were observed, suggesting that this core catcher sample belongs to lower Zone NN4 (CN3).
Several lower Miocene samples investigated from below Section 199-1218A-5H-CC contain common to abundant sphenoliths but no S. heteromorphus or Sphenolithus belemnos. Sediment deposits with an age range of over 7 m.y. may be contained in Cores 199-1218A-5H and 6H. Available calcareous nannofossil data cannot resolve if a hiatus is present or if condensation was a factor.
O/M Boundary Interval
Several species events are employed in the classical zonal schemes to subdivide the interval around the O/M boundary, which is recognized by the presence of S. delphix in the upper portion of Core 199-1218A-10H. Three other events are commonly used to subdivide the lowermost Miocene. These include the base of Discoaster druggii (observed in the lower part of Section 199-1218B-9H-5), the base of Sphenolithus disbelemnos (observed in Section 199-1218A-9H-4), and the top of Triquetrorhabdulus carinatus (observed in the core break between Cores 199-1218A-6H and 7H). Detailed distribution patterns of these species at the Oligocene-Miocene transition are shown in Figure F8. S. disbelemnos provides a distinct biostratigraphic signal just above the O/M boundary, in contrast to the basal part of the range of D. druggii, which is characterized by discontinuous occurrences of few to rare specimens. Triquetrorhabdulus milowii is observed in a short interval immediately above the range of T. carinatus. Two biohorizons are recognized within the upper range of T. carinatus, based on the pattern observed between Cores 199-1218A-7H and 9H and Cores 199-1218B-7H and 10H (Fig. F8). The interval between Sections 199-1218A-7H-5 and 9H-2 yields only discontinuous, rare specimens of T. carinatus, which becomes an abundant member of the assemblage in Sample 199-1218A-9H-2, 130 cm, and downhole for a long interval into the upper Oligocene.
Three species events are used to subdivide the uppermost Oligocene. These include the top of common to abundant Cyclicargolithus abisectus (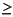 10 Ám) in Section 199-1218A-11H-5, the top of Sphenolithus ciperoensis in Section 11H-3, and the top of Dictyococcites bisectus in Section 13H-6. C. abisectus provides a distinct event, whereas D. bisectus and S. ciperoensis are rare members of the assemblages in the uppermost part of their ranges, implying that their true extinctions are difficult to determine accurately. Specimens of C. abisectus in Section 199-1218A-7H-CC are considered to be reworked from Oligocene strata (Fig. F8).
10 Ám) in Section 199-1218A-11H-5, the top of Sphenolithus ciperoensis in Section 11H-3, and the top of Dictyococcites bisectus in Section 13H-6. C. abisectus provides a distinct event, whereas D. bisectus and S. ciperoensis are rare members of the assemblages in the uppermost part of their ranges, implying that their true extinctions are difficult to determine accurately. Specimens of C. abisectus in Section 199-1218A-7H-CC are considered to be reworked from Oligocene strata (Fig. F8).
S. ciperoensis overlaps with abundant C. abisectus for a short interval in Core 199-1218A-11H, causing Subzone CN1a to disappear. A similar relationship between these two species events is observed on the Ontong Java Plateau in the western equatorial Pacific Ocean (Kroenke, Berger, Janecek, et al., 1991) and the Ceara Rise in the western equatorial Atlantic Ocean (Curry, Shackleton, Richter, et al., 1995).
Of the discussed lower Miocene-upper Oligocene calcareous nannofossil events, only three are well calibrated to an independent chronology: namely, the base of S. disbelemnos at 22.7 Ma, the top of S. delphix at 23.0 Ma, and the base of S. delphix at 23.2 Ma (Shackleton et al., 1999).
Oligocene
Oligocene assemblages are generally monotonous in composition apart from the evolution observed among the sphenoliths. Helicosphaerids are absent in Miocene and upper Oligocene sediments at Site 1218, probably because of dissolution, but are rare to few in the assemblages of the lower Oligocene and are notably represented by Helicosphaera compacta.
Key elements of upper lower Oligocene (NP23/CP17-18) assemblages are abundant (C. floridanus, D. bisectus, and Sphenolithus moriformis) few to common (Coccolithus pelagicus, Coccolithus eopelagicus, Dictyococcites hesslandii [junior synonym: Dictyococcites scrippsae], and D. deflandrei), and few to rare (Sphenolithus predistentus, Sphenolithus celsus, Reticulofenestra gartneri, Discoaster tanii, and H. compacta).
The NP23/NP22 (CP17/CP16c) boundary is distinct in Section 199-1218A-22X-2, occurring over a 77-cm-long interval. Core 199-1218A-22X also contains unusually large specimens of D. bisectus, similar in size to the large (per definition) Reticulofenestra umbilicus. The NP22/NP21 (CP16c/CP16a+b) boundary is equally distinct, occurring over a 35-cm interval in the uppermost part of Section 199-1218A-23X-3.
E/O Boundary Interval
The extinction of the planktonic foraminifer genus Hantkenina is adopted for recognition of the E/O boundary. The nearest calcareous nannofossil event is the extinction of D. saipanensis, estimated to have occurred ~0.3 m.y. prior to the Hantkenina event and, thus, the E/O boundary. The last representative of the Paleogene rosette-shaped discoasters, D. barbadiensis, disappeared ~0.2 m.y. before D. saipanensis.
D. barbadiensis disappeared over a 30-cm interval in Section 199-1218A-24X-5, and D. saipanensis disappeared over a 22-cm interval in Section 24X-4, shortly below the major change in lithology. The lithology change occurs in two steps, from radiolarite below to nannofossil chalk above, observed in Cores 199-1218A-24X and 199-1218C-17X, respectively. A complete composite stratigraphic section is obtained from these cores, revealing that the entire two-step change in lithology occurs within Zone NP21 (CP16a and CP16b) above the extinction of the last Eocene discoasters. By assuming an LSR within Zone NP21 in this composite section, an age estimate of 33.3 Ma is obtained for the initial change (midpoint of transition) in lithology and an estimate of 32.9 Ma for the midpoint of the second, final step. The boundary condition change of the ocean-climate system that caused the first step of this drastic deepening of the CCD and the accompanying change in sedimentation in the tropical Pacific Ocean occurred in middle Oi-1 (33.5-33.1 Ma) (Zachos et al., 2001) on the common timescale of Cande and Kent (1995).
Rare specimens of the "cool-water marker" (Bukry, 1973), or "nontropical species" in Martini's (1971) vocabulary, Isthmolithus recurvus were only observed in Samples 199-1218A-24X-1, 1 cm, and 17X-CC, perhaps indicating brief episodes of cooler surface water conditions.
Eocene
The number of species preserved and the total abundance of calcareous nannofossils were strongly biased by calcite dissolution in the upper Eocene and upper middle Eocene radiolarites. Typical remaining forms included C. pelagicus, C. eopelagicus, D. barbadiensis, D. saipanensis, D. tanii, Discoaster nodifer, D. bisectus, D. hesslandii, Ericsonia formosa, R. umbilicus, Reticulofenestra dictyoda, and R. gartneri. Three middle Eocene species events were determined in the interval, which show enhanced dissolution: the top of Chiasmolithus grandis, the base of D. bisectus, and the top of Chiasmolithus solitus.
Sphenoliths reappeared in the better-preserved, lowermost interval of Hole 1218A and included Sphenolithus furcatolithoides and Sphenolithus radians, both becoming extinct in middle Eocene Subzone CP14a (NP16). The assemblage of large reticulofenestrids is characterized by higher abundances of morphotypes <14 Ám in the lowermost sample of the sequence (Sample 199-1218A-30X-CC). The species R. umbilicus (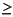 14 Ám) is still present in that sample, albeit as a rare member of the nannofossil assemblage. The uppermost occurrence of Nannotetrina spp. is also observed in Section 199-1218A-30X-CC, indicating that this section was formed during Zone NP16 close to the CP14a/CP13 boundary.
14 Ám) is still present in that sample, albeit as a rare member of the nannofossil assemblage. The uppermost occurrence of Nannotetrina spp. is also observed in Section 199-1218A-30X-CC, indicating that this section was formed during Zone NP16 close to the CP14a/CP13 boundary.
Planktonic foraminifers are present sporadically through the lower Miocene, Oligocene, and middle Eocene at Site 1218 with generally less consistent occurrence than other calcitic groups such as benthic foraminifers and calcareous nannofossils. Most samples show at least some dissolution of planktonic foraminifers, with preferential preservation of thick-walled, large specimens in many cases. Middle Oligocene sediments (Zones P21 and P20) are generally the best preserved and most species rich, whereas the Pleistocene-middle Miocene and lower Oligocene could not be zoned at all owing to the absence of dissolution-susceptible marker species (Table T4). Planktonic foraminifers were completely absent in samples within the upper Eocene. The distribution of middle Eocene species is given in Table T5. Well-defined datum levels for planktonic foraminifers are presented in Table T6.
Planktonic foraminifers can be used to delineate a condensed sequence of lower Miocene Zones M1a-M3 in Holes 1218A and 1218B (Fig. F7). Core catchers from Hole 1218A contain planktonic foraminiers in Sample 199-1218A-5H-CC (46.35 mbsf) that include an undifferentiated Miocene assemblage of Paragloborotalia mayeri, Paragloborotalia continuosa, and Paragloborotalia siakensis. The top of Zone M3 is located in Sample 199-1218A-6H-7, 39-41 cm, which contains the highest occurrence of Catapsydrax dissimilis ciperoensis. The M2/M3 boundary could not be differentiated at Site 1218 owing to the absence of Globigerinatella insueta. Zone M2/M3 assemblages are present as deep as Section 199-1218A-7H-CC and are characterized by dissolution-resistant species such as Globoquadrina praedehiscens, Globoquadrina venezuelana, P. mayeri, and Globorotaloides suteri. The highest occurrence of Paragloborotalia kugleri is in Sample 199-1218A-8H-2, 110-114 cm (67.8 mbsf), and defines the top of Subzone M1b. Subzone M1a is present between Sections 199-1218A-8H-6, 60-62 cm, and 10H-1, 130-132 cm, as recognized, respectively, by the disappearance of Globoquadrina dehiscens and the first appearance of P. kugleri. The corresponding interval in Hole 1218B is indicated by the last downhole occurrence of G. dehiscens in Sample 199-1218B-9H-4, 63-65 cm. The base of Subzone M1a, and, therefore, the M/O boundary, is placed between Samples 199-1218A-10H-1, 130-132 cm, and 10H-2, 74-75 cm (86.15 ▒ 0.30 mbsf).
Catapsydrax dissimilis displays two distinctive morphologies in the Oligocene-lowermost Miocene section at Hole 1218A. One form closely resembles the holotype of this species; it possesses 3.5 large inflated chambers and a thin, straplike bulla over the aperture. The other form is similar to the holotype specimen of C. dissimilis ciperoensis, named by Blow and Banner (1962), and specimens figured as C. dissimilis by Kennett and Srinivasan (1983). Specimens we refer to C. dissimilis ciperoensis have four slightly compressed chambers and an aperture completely covered by a flattened bulla that forms two slitlike secondary apertures. Our studies suggest that C. dissimilis (sensu stricto) ranges only to the top of Subzone M1a at Site 1218, whereas C. dissimilis ciperoensis is the form that ranges to the top Zone M3 and defines the M3/M4 zonal boundary.
A number of long-ranging Miocene species have first occurrences in the basal Miocene sequence at Site 1218, although several characteristic species of the Oligocene range into the lower Miocene. Dentoglobigerina altispira, P. continuosa, G. praedehiscens, and Globoquadrina biniaensis have first occurrences within Zone M1 in Hole 1218A. Species typical of the Oligocene, such as Globoquadrina sellii, Paragloborotalia pseudokugleri, Globoquadrina tripartita, and Subbotina gortani, were all found in the lower Miocene at this site. The Miocene ranges of G. sellii, G. tripartita, and S. gortani are unusual and when coupled with the occurrence of these taxa in the Oligocene of Site 1218 suggest reworking from Oligocene strata.
Assemblages of Zone P22 are present between Sections 199-1218A-10H-2 and14H-4 (86.9-127.01 mbsf). Preservation is mostly very poor in this interval, with only dissolution-resistant species present in Cores 199-1218A-10H, 11H, and 13H. Characteristic species include Globoquadrina tapuriensis, G. tripartita, G. suteri, and, in the lower part, Paragloborotalia opima nana.
Subzone P21b is recognized by the highest occurrence of Paragloborotalia opima opima in Sample 199-1218A-14H-5, 32-37 cm (128.52 mbsf), and the first common occurrence of Chiloguembelina cubensis in Sample 16H-7, 39-44 cm (150.59 mbsf). This interval includes the best-preserved planktonic foraminifer assemblages in the whole of Site 1218. The zone marker, P. opima opima, is present continuously and is frequently the most abundant species. Therefore, the top of Subzone P21b is very well delineated. In contrast, C. cubensis is very rare in Hole 1218A and may not be useful as a datum level. Subzone P21b includes G. tapuriensis (including specimens with an inflated, subspherical final chamber), G. tripartita, G. praedehiscens, G. praeturritilina, Subbotina euapertura, Catapsydrax dissimilis, Tenutiella clemincea, and Tenutellinata angustiumbilicata in the >150-mm size fraction. Also present at low abundance levels is Globoquadrina prasaepis, a relatively large four-chambered taxon that is similar to S. euapertura but differs from it by having a more compressed final chamber and a more restricted, lower aperture. Large and typical forms of G. venezuelana, which are consistently present throughout the lower Miocene and upper Oligocene, disappear below Core 199-1218A-15H from this site. It is of note that a number of the forms referred to C. dissimilus ciperoensis are extraordinarily large and inflated for the taxon.
The assemblages from Samples 199-1218A-16H-CC to 18H-4, 98-103 cm, are assigned to the P20/P21 zonal range. This interval is delineated at its base by the downhole first occurrence of Turborotalia ampliapertura in Sample 199-1218A-18H-4, 98-103 cm. The assemblages are less well preserved than in Subzone P21b and only occasionally contain G. tapuriensis and G. tripartita. The last downhole occurrence of five-chambered low trochospiral species, including P. mayeri, is present in Sample 199-1218A-17H-CC. Globigerina angulisuturalis was not found in the samples from Site 1218, and therefore, we are unable to differentiate Zone P20 from Subzone P21a.
Planktonic foraminiferal assemblages become increasingly dissolved and impoverished from Sample 199-1218A-19H, 98-103, through 23X-CC, coincident with the shift from the nannofossil ooze to chalk at the base of lithostratigraphic Unit II. Based on the occurrence of Turborotalia ampliapertura and a lack of characteristic Eocene forms, we assign this interval to the P18/P19 zonal range. The assemblages from this interval are composed of dissolution-resistant species, with Catapsydrax dissimilis, G. suteri, Paragloborotalia nana, G. tripartita, and Tenutellinata angustiumbilicata occurring most consistently. Catapsydrax unicavus is also present in Samples 199-1218A-18H-5, 98-100 cm, to 20H-2, 102-104 cm, but disappears below this. S. euapertura, which ranges from the top of Core 199-1218A-15H, has its last common downhole occurrence in Sample 199-1218A-19H-4, 100-105 cm. Subbotina angioporoides and S. utilisindex are also present in the middle part of Core 199-1218A-20H. We do not find Pseudohastigerina micra, the marker species for the base of the Oliogocene, in any of the samples from Site 1218 and are, therefore, unable to identify the lower boundary of Zone P19. Dissolution may be responsible for the absence of most foraminifers other than heavily encrusted species from the <150-Ám fraction below Core 199-1218A-20H. Samples 199-1218A-22X-2, 28-32 cm; 22X-1, 40-45 cm; and 23X-CC contain a species that is very similar to, but slightly smaller than, P. opima opima (to which it has been referred) and not as small as co-existing P. opima nana. This is an unusually low occurrence for P. opima opima and suggests contamination of younger material downhole. However, the small size of these specimens indicates that they may represent an in situ but, as yet, unidentified species that is closely related to P. opima opima s.s.
The E/O boundary interval, which occurs in Core 199-1218A-24X and the subjacent core (25X) contains well-preserved benthic foraminifers but is barren of planktonic foraminifer species. Thus, we were unable to identify the end Eocene event, as denoted by the last occurrence of Hantkenina (Coccioni et al., 1988). The bottom four cores of Hole 1218A (199-1218A-26X through 29X) contain sparse and moderately well-preserved dissolution-resistant planktonic foraminifers of middle Eocene age. The assemblages are composed mainly of parasubbotinids, paragloborotalids, and the broken, yet distinctive, elongate chambers of various species of the genus Clavigerinella. Species include small and compact Parasubbotina griffinae, Paragloborotalia nana, a form comparable to "H." cf. bolivariana (Toumarkine and Luterbacher, 1985; fig. 27, p. 126), Clavigerinella jarvisi, C. eocanica, C. colombiana, C. akersi, Catapsydrax unicavus, and Muricoglobigerina senni. Species of the genera Morozovella and Globigerinatheka are not present, which makes precise dating of these assemblages problematic. The occasional occurrence of Acarinina bullbrooki, A. rohri, A. cf. collactea, and A. punctocarinata indicate a middle Eocene age (probably equivalent to Zones P11 and P12) and a minimum age of 40.5 Ma (last occurrence datum of A. bullbrooki). Also present in these assemblages is a heavily calcified three-chambered form, here referred to as Parasubbotina cf. linaperta. As the name suggests, this species resembles Subbotina linaperta in general morphology but has a more prominent apertual lip and a wall texture that is more closely comparable to that of Parasubbotina or Paragloborotalia.
Similar assemblages containing unusually large numbers of parasubbotinids and clavigerinellids, commonly occurring with radiolarian-rich sediments, have been found at ODP Sites 959 and 960 (Ivory Coast), Kane 9-C piston core (Endeavour Seamount), and onshore sections in Ecuador, Peru, Colombia, and California. This restricted distribution pattern suggests that their presence may be linked to conditions of high productivity due to upwelling during the Eocene. The occurrence of Clavigerinella and Parasubbotina at Site 1218, which is believed to have been located within the equatorial productivity belt during the middle Eocene, further supports this view.
Benthic foraminifers are present throughout the cored interval at Site 1218 (Table T7), except for core catcher Samples 199-1218A-24X-CC through 27X-CC and 1H-CC through 4H-CC, which are barren of benthic foraminifers. The assemblages examined are predominantly composed of well-preserved and diverse calcareous forms, and agglutinated foraminifers are rare and poorly preserved (Fig. F9).
Benthic foraminifers such as Siphonodosaria abyssorum, Oridorsalis umbonatus, Pullenia subcarinata, and various forms of dentalinids/nodosarids are consistently present in all samples. These species are long ranging (Eocene-Holocene) and of little stratigraphic use. Species of the genera Globocassidulina, Gyroidinoides, and Cibicidoides are also common in most samples at this site. Species of the genus Cibicidoides, such as C. havanensis, C. grimsdalei, and C. praemundulus, also have long stratigraphic ranges and are present from the middle Eocene to the middle Miocene. They are indicators of lower bathyal and abyssal palaeoenvironments but are also commonly reported from abyssal paleodepths (van Morkhoven et al., 1986). These assemblages indicate lowermost bathyal and upper abyssal paleodepths at this site during the middle Miocene and Oligocene.
Nuttallides umbonifer is present but rare in middle Miocene-Oligocene sediments between Samples 199-1218A-16H-CC and 23X-CC and is sporadically present between 5H-CC and 15H-CC. This species is tolerant of low food supply and is adapted to waters that are undersaturated with respect to calcite (Nomura, 1995). O. umbonatus is present in all the samples where foraminifers are present. C. praemundulus is sporadically present between Samples 199-1218A-10H-CC and 30X-CC. O. umbonatus is considered to be adapted to sediments with low organic carbon (Corg)content. Cibicidoides mundulus, which is the descendant of C. praemundulus, is also common in areas of low productivity or low Corg content (Nomura, 1995). This information suggests that little organic material was received at the seafloor in this deepwater setting. Globocassidulina spp. are very common in Sections 199-1218A-9H-CC to 12H-CC. Some of these taxa are similar to Globocassidulina subglobosa, which is sometimes reported as preferring environments with high Corg content. However, there are taxonomic problems associated with the identification of this species, so we do not utilize these taxa to interpret the depositional environment. In contrast, Epistominella exigua indicates the influence of high productivity at other times. This species, which is long ranging in most deep-sea sites, is restricted to Sections 199-1218A-8H-CC and 9H-CC.
Samples 199-1218A-24X-CC through 27X-CC contain very poorly preserved foraminiferal assemblages that are characterized by large specimens of C. grimsdalei, C. eocaenus, Siphionodosaria abyssorum, and O. umbonatus.
Nuttallides truempyi, which is commonly found in Samples 199-1218A-28X-CC and 29X-CC, is present in association with Cibicidoides eocaenus, C. grimsdalei, C. praemundulus, and Anomalinoides spissiformis. N. truempyi commonly disappears at the end of the middle Eocene (Berggren and Miller, 1989; Nomura, 1995); thus, Samples 199-1218A-28X-CC and 29X-CC can be assigned to the middle Eocene. The assemblage composition indicates lower bathyal to abyssal paleodepths.
In order to evaluate the preservation of foraminiferal test walls more thoroughly than is possible under reflected light, optical textures of a number of species were examined under transmitted light: O. umbonatus in Samples 199-1218A-10H-CC, 17H-CC, 22H-CC, and 28H-CC; Globocassidulina sp., Gyrodinoides sp., and P. subcarinata in Sample 199-1218A-10H-CC; and Fursenkoina sp., Fissurina sp., and N. truempyi in Sample 199-1218A-28X-CC. Fissurina sp. and N. truempyi show a well-defined radial texture (Fig. F9A) that indicates the original crystalline structure is preserved. Other species, which have a granular texture, also retain an original crystalline structure (Fig. F9B). These observations indicate that most of the benthic foraminifers at this site have suffered little or no postburial diagenetic alternation. The exceptions to this are Samples 199-1218A-24X-CC through 27X-CC and 29X-CC through 30X-CC, in which benthic foraminifers show signs of dissolution.
Radiolarians were found in all recovered material. The stratigraphic distribution of radiolarian datums is shown in Table T8. The fauna in Samples 199-1218A-1H-1, 45-47 cm, to 1H-3, 46-48 cm, consists of moderately well preserved and relatively rare Pleistocene radiolarians belonging to Zone RN15. Sample 199-1218A-1H, 45-47 cm, contains a similar fauna belonging to Zone RN14. Sections 199-1218A-1H-5 through 2H-7 contain a mixed assemblage of sparse and poorly preserved Eocene-Miocene radiolarians, for which we were unable to assign an age. These mixed assemblages may be the result of slumping or unusual current activity. Sample 199-1218A-2H-CC is tentatively assigned to Zone RN10.
The quality and quantity of the radiolarian fauna improves somewhat in Sample 199-1218A-3H-4, 46-48 cm (Zone RN7), is variable throughout the late Miocene and late middle Miocene assemblages found in Cores 199-1218A-4H and 5H, and is generally common to abundant and well preserved in the rest of the downhole material. The boundary between Zones RN7 and RN6 lies between Samples 199-1218A-4H-3, 45-47 cm, and 4H-5, 45-47 cm. The latter sample contains an abundance of monospecific, D-shaped sagittal rings. The boundary between Zones RN6 and RN5 lies between Sample 199-1218A-4H, 45-47 cm, and Section 4H-CC, based on the absence of Diartus petterssoni in the latter sample. The boundary between Zones RN5 and RN4 lies between Samples 199-1218A-5H-4, 45-47 cm, and 5H-5, 45-47 cm. The first occurrence of Calocyclotta costata in Sample 199-1218A-6H-1, 45-47 cm, places the RN4/RN3 boundary between Samples 199-1218A-6H-1, 45-47 cm, and 6H-2, 45-47 cm, whereas the first occurrence of Stichocorys wolffii places the RN3/RN2 zonal boundary between Samples 199-1218A-6H-7, 45-47 cm, and 6H-CC. The earliest Neogene zone (RN1) is found in Sample 199-1218A-7H-7, 46-48 cm, and continues through 9H-5, 74-75 cm.
The O/M boundary is approximated here by the range of calcareous nannofossil S. delphix between Subchrons C6Cn.3n and C6Cn.2n (Raffi, 1999) and the first occurrence of the planktonic foraminifer P. kugleri at the same level and lies within the upper part of radiolarian Zone RP22. This latest Paleogene radiolarian zone is found between Samples 199-1218A-9H-6, 74-75 cm, and 11H-3, 44-46 cm, and is marked by the presence of Lychnocanoma elongata and the absence of Cyrtocapsella tetrapera. For radiolarian biostratigraphy, the first occurrence of C. tetrapera serves as a good approximation to the O/M boundary (Sanfilippo and Nigrini, 1995).
Below Sample 199-1218A-11H-3, 44-46 cm, there is an expanded Oligocene comprised of only two zones (RP21 and RP20). The boundary between these zones, which is defined by the evolutionary transition from Tristylospyris triceros to Dorcadospyris ateuchus, is between Samples 199-1218A-18H-6, 46-48 cm, and 18H-CC. The section between 199-1218A-19H-CC and 23X-CC is also rich in diatoms.
The top of the next older radiolarian zone (RP19) is thought to approximate the E/O boundary and is present in Hole 1218A between Samples 199-1218A-23X-CC and 24X-1, 45-47 cm. The upper limit of this zone was further constrained using material from Hole 1218C and was found to lie between Section 199-1218A-23X-CC (238.58 mcd) and Sample 199-1218C-17X-4, 56-58 cm (238.96 mcd). The lithologic change from nannofossil chalk to radiolarite is placed at 241.98 mcd. Zone RP19 extends to Section 199-1218A-24X-CC. Upper Eocene Zone RP18 is represented in only one sample (199-1218A-25X-1, 24-26 cm). The rest of the hole is of middle Eocene age with Zone RP17 extending from Sample 199-1218A-25X-2, 82-24 cm, through 6X-1, 46-48 cm; Zone RP16 from 26X-2, 45-47 cm, through 27X-1, 45-47 cm; and Zone RP15 from 27X-2, 45-47 cm, through 28X-4, 46-48 cm, at least. Sections 199-1218A-28X-CC through 29X-3, 49-51 cm, belong to either Zone RP15 or RP14. Trace occurrences of radiolarians in the rest of Cores 199-1218A-29X and 30X contain no age diagnostic species.
A limited number of samples were taken from Holes 1218B and 1218C in order to further constrain some first and last occurrences of species and zonal boundaries (Table T8).

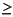 10 Ám) in Section 199-1218A-11H-5, the top of Sphenolithus ciperoensis in Section 11H-3, and the top of Dictyococcites bisectus in Section 13H-6. C. abisectus provides a distinct event, whereas D. bisectus and S. ciperoensis are rare members of the assemblages in the uppermost part of their ranges, implying that their true extinctions are difficult to determine accurately. Specimens of C. abisectus in Section 199-1218A-7H-CC are considered to be reworked from Oligocene strata (Fig. F8).
10 Ám) in Section 199-1218A-11H-5, the top of Sphenolithus ciperoensis in Section 11H-3, and the top of Dictyococcites bisectus in Section 13H-6. C. abisectus provides a distinct event, whereas D. bisectus and S. ciperoensis are rare members of the assemblages in the uppermost part of their ranges, implying that their true extinctions are difficult to determine accurately. Specimens of C. abisectus in Section 199-1218A-7H-CC are considered to be reworked from Oligocene strata (Fig. F8).