DISCUSSION
Site 1224 ferrobasalts are among the most differentiated basalts sampled in the Pacific Ocean crust. They have anomalously high concentrations of incompatible immobile trace elements (e.g., Nb, Zr, Hf, Ta, and Y) (Haraguchi and Ishi, this volume). Lustrino et al. (2004) concluded that Site 1224 basalt formed by variable extents of melting of a typical mid-ocean-ridge basalt (MORB) source, but their highly evolved compositions reflect extensive polybaric fractionation of plagioclase, olivine, and clinopyroxene.
Geothermometry
Using the Beattie (1993) olivine-glass geothermometer, eruptive temperatures were 1147°–1167°C. Using the graphic pyroxene geothermometer of Lindsley and Andersen (1983), pyroxene both near pillow rims that formed at high undercooling and in flow interiors at lesser undercooling began to crystallize from near these temperatures to as low as 1000°C. Titanomagnetite intergrowth with pyroxene began crystallization at ~1050°C, thereby lowering TiO2 contents in clinopyroxene as crystallization proceeded. At lower temperatures, ilmenite began to crystallize as well. Among the pillow basalts, the clinopyroxenes in each basalt type crystallized within 50°–70°C of the liquid temperatures. In the most slowly cooled interior of the upper flow, the crystallization interval spanned ~130°C, with the most extensively differentiated clinopyroxene occurring in the sample with myrmekitic intergrowths adjacent to plagioclase.
Two-oxide geothermometry (Frost and Lindsley, 1991; Frost et al., 1988) indicates that ilmenite and magnetite crystallized together at ~850°–900°C at an oxygen fugacity about one log unit below the nickel/nickel oxide buffer (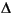 NNO). Most titanomagnetite, however, was strongly affected by low-temperature reaction with hydrothermal fluids, losing much of its magnetite (Mt) component to dissolution while retaining ulvöspinel. This may partly have provided the oxidized iron that is in the iron oxyhydroxide veins and adjacent alteration haloes.
NNO). Most titanomagnetite, however, was strongly affected by low-temperature reaction with hydrothermal fluids, losing much of its magnetite (Mt) component to dissolution while retaining ulvöspinel. This may partly have provided the oxidized iron that is in the iron oxyhydroxide veins and adjacent alteration haloes.
Origin of the Quartz-Sodic Plagioclase Intergrowth
Small amounts of felsic rock (e.g., quartz-diorites, tonalites, and trondhjemites) are found in Layer 3 of the oceanic crust and are classically referred to as oceanic plagiogranites (e.g., Thayer, 1977; Aldiss, 1981). Such evolved rocks have been drilled in several Ocean Drilling Program (ODP) sites and in plutonic sections of ophiolites (see Koepke et al., 2004, for a detailed list of occurrences). Many of these plagiogranites show particular microstructures called myrmekites. The term myrmekite designates the "wartlike" appearance of quartz-plagioclase intergrowths seen in thin sections of plutonic rocks (Sederholm, 1897). The term has usually been applied to intergrowths of quartz and sodic plagioclase in rocks with essential K-feldspar, such as granites and gneisses (Barker, 1970). However, reaction rims on calcic plagioclase in basaltic rocks (Pavlov and Karskii, 1949) and main zone gabbroic cumulates of the Bushveld intrusion (Wager and Brown, 1967) have also been termed myrmekite.
The intergrowths at Site 1224 are similar in bulk composition to the myrmekites in granites (Barker, 1970) and also to dikelets of plagiogranites found in abyssal gabbros from the Southwest Indian Ridge and eastern Pacific (Koepke et al., 2004, and references therein).
The origin of plagiogranites is hotly debated, and three primary models have been proposed: (1) protracted differentiation by crystal fractionation of a MORB parental magma (e.g., Juster et al., 1989; Toplis and Carroll, 1995; Niu et al., 2002), (2) partial melting of oceanic gabbros in high-temperature shear zones (e.g., Cortesogno et al., 2000; Dick et al., 2000; Koepke et al., 2004), and (3) liquid immiscibility (e.g., Dixon and Rutherford, 1979; Natland et al., 1991).
The myrmekitic intergrowths at Site 1224 have higher SiO2 and lower FeOtot than silicic glasses from the EPR, including a dacite glass bleb found in a ferroandesite dredged from the eastern limb of the 9°N nontransform offset of the East Pacific Rise (Natland, 1991), which is the most siliceous glass thus far analyzed from the ocean basins (Fig. F7A). Moreover, compared to EPR silicic glasses, they have higher Cross, Iddings, Pirsson, and Washington (CIPW) normative quartz (Fig. F7B) and much lower normative orthoclase (Fig. F7C). The normative plagioclase composition of the myrmekitic intergrowths is An8–10 (Fig. F7B, F7C). The experimental partial melts of cumulate gabbros cored from ODP Legs 176 (Site 735B; Southwest Indian Ridge) and 153 (Mid-Atlantic Ridge Kane Fracture Zone) obtained by Koepke et al. (2004) show compositions very different from those of myrmekites. In particular, Figure F7 shows the lower SiO2, lower CIPW normative quartz, and higher FeOtot content of the experimental melts of Koepke et al. (2004) compared to the myrmekitic intergrowths at Site 1224; other differences (not shown) are the higher Al2O3 and CaO and lower Na2O contents of the experimental melts. In the ternary normative diagram representing the granitic portion of petrogeny's residua system (Fig. F7D), two of the three intergrowth compositions from Site 1224 plot nearly at the sideline quartz-albite binary eutectic at a vapor pressure of ~500 atm (0.506 Kbar), consistent with formation at or near the seafloor. The binary eutectic at this point is at ~870°C (Tuttle and Bowen, 1958), a value similar to the temperature estimates for late-stage liquids using coexisting ilmenite and magnetite. The other sample plots toward higher normative quartz. An immiscible dacite globule from dolerite at Deep Sea Drilling Project Site 396, on the eastern flank of the Mid-Atlantic Ridge (Sato, 1979), plots nearby but is somewhat more potassic than the intergrowths. Siliceous glasses segregated experimentally from ferrobasalt liquids (Dixon and Rutherford, 1979) do not fall on any of the three-phase cotectic boundaries plotted in Figure F7D but instead have significantly higher normative quartz. The experimental melts of Koepke et al. (2004) show large compositional variation, with values overlapping the composition of the myrmekites of Site 1224 to compositions with much lower quartz (Fig. F7D). Note the overlap between the plagiogranites found in natural samples in Hole 735B and the experimental melts of Koepke et al. (2004), which used Hole 735B gabbros as starting material.
We conclude that the Site 1224 intergrowth compositions are not those of liquids. The projected phase relations suggest instead that they are a cryptocrystalline intergrowth of sodic plagioclase and quartz that precipitated from liquids that plausibly resembled the dacitic natural glass or some of the experimental immiscible siliceous liquids, these liquids being in particular more potassic and more siliceous than the intergrowths. An immiscible origin for the liquids that produced the intergrowths is supported by the lack of a continuum in plagioclase compositions between groundmass plagioclase (~An36) and the normative plagioclase compositions (An8–10) of the intergrowths, the sharp boundaries of the intergrowths with adjacent plagioclase, and their colloform shapes where they extend convexly into the mesostasis.
Other mechanisms of formation are possible. Orville (1963) described an ion-exchange reaction between albite (NaAlSi3O8) and a solution with excess CaCl2 that produced anorthite (CaAl2Si2O8) with tiny inclusions of quartz, but he was unable to produce a similar result by reaction between anorthite and a solution with excess NaCl, evidently because of kinetic effects (Barker, 1970, citing a personal communication from P. Orville). Despite this negative result, these experiments do not preclude the possibility that in a more complex natural system, intergrowths of quartz and sodic plagioclase may form by reaction between late-stage magmatic or hydrothermal brines and minerals that crystallized interstitially from highly differentiated, but not necessarily granitic, liquids. Nevertheless, whether it is by direct crystallization differentiation, liquid immiscibility, or metasomatic process, granitic microenvironments clearly develop intersertally even in the coarse-grained flow interiors of parental abyssal tholeiites.
The uniform chemical compositions of least-altered whole rocks show that the intersertal granitic microenvironments remained where they formed; they did not coalesce or concentrate into veins or veinlets in accord with Marsh's (1995) observation that aphyric basaltic liquids lack a mechanism such as buoyancy-driven separation of phenocrysts from liquids to segregate minerals. Only interstitial differentiation occurs in aphyric flows or intrusions, and this does not appreciably affect the bulk compositions of analyzed rocks. A corollary is that formation of veins of tonalite or trondhjemite in abyssal gabbros and even differentiation of basalt types, as exemplified by the short section of basalts cored at Site 1224, requires some additional means of separating crystals from residual liquids. At slow-spreading ridges, the apparent mechanism is expulsion of residual liquids from a crystalline matrix by a combination of compactive and shear deformation (Natland and Dick, 2001), what Bowen (1920) termed "differentiation by deformation." Based on the little-deformed gabbros sampled at Hess Deep in the eastern Pacific, the agency of separation of residual liquids from cumulates beneath fast-spreading ridges is evidently more strongly dominated by compaction than by differential horizontal stress (Natland and Dick, 1996).
Figure F8 summarizes the extended liquid line of descent for abyssal tholeiite magma, as deduced from mineral relations seen at Site 1224 and the gabbro suites from the eastern Pacific. An initial primitive basaltic liquid first crystallizes olivine and plagioclase, with clinopyroxene soon joining the liquidus. This results in a trend of enrichment in FeOtot and TiO2 until at ~4%–5% MgO content and 1080°–1050°C, where titanomagnetite joins plagioclase and clinopyroxene in the crystallization sequence. Iron enrichment persists as crystallization differentiation proceeds, but TiO2 enrichment is retarded by titanomagnetite crystallization until ~1000°C, where two silicate liquids, one very iron rich and the other siliceous, separate immiscibly. The separate liquids each internally continue to differentiate on their own, with ilmenite and titanomagnetite coprecipitating from the more iron rich liquid and quartz and sodic plagioclase from the silica-rich liquid to produce dacitic and even truly rhyolitic residua.
Along the EPR, nearly two dozen lavas of generally andesitic composition have been sampled at different places. In hand specimens they are extremely dark rocks, black compared with the gray of basalts, and this is because they are still sufficiently rich in FeOtot and TiO2 that they crystallize abundant titanomagnetite. They could accurately be termed ferroandesite or even icelandite to distinguish them from the light gray and not nearly as iron-rich calc-alkalic andesite so common in island arcs. If the usual liquid line of descent along the EPR is like that just described and involves late-stage liquid immiscibility, then all andesites from this spreading ridge must be hybrid rocks, mixes between dacitic to rhyodacitic liquids and evolved but nevertheless still common basalt with between 2% and 3% TiO2 content (double-headed arrows in Fig. F8). The andesites are not simply a continuation of the basaltic liquid line of descent turned abruptly toward lower TiO2 contents by the onset of crystallization of titanomagnetite and ilmenite. A composite dredge sample having an andesite interior and basalt carapace, obtained from the eastern limb of the 9°N nontransform offset of the EPR, provided clear mineralogical evidence for such mixing (Natland, 1991). Evidence for similar mixing along the Galapagos Spreading Center was presented by Juster et al. (1989).
Opaque Minerals Composition
The high ulvöspinel content of the titanomagnetites is a surprise, being quite unlike compositions seen in fresh basalt with a wide range of differentiation dredged from the EPR (Gee and Kent, 1997) or even somewhat altered basalt drilled along the Mid-Atlantic Ridge (Johnson and Melson, 1979). The latter rocks contain cation-deficient titanomaghemite altered after original titanomagnetite. The cation-deficient titanomaghemite, however, differs only slightly from the fresh mineral in its proportion of magnetite to ulvöspinel and does not approach the deficiency in magnetite found in the spinel that we analyzed from Site 1224 (Fig. F4). The near uniformity of unaltered titanomagnetite in fresh basalt representing different stages of differentiation (i.e., from olivine tholeiites to ferrobasalt) clearly stems from the common composition along a liquid line of descent where this mineral joins the liquidus.
With only a pair of adjacent ilmenites analyzed in a single sample from Site 1224, it is not possible to determine if ilmenite composition correlates with titanomagnetite composition. The two grains lie at the ilmenite-rich, hematite-poor end of the spectrum among basalts and dikes and are similar in composition to many ilmenite grains in abyssal gabbros (Dick et al., 2002).
In general, variations in composition of both ilmenite and magnetite result from oxyexsolution, the first stages of which produce lamellae of ilmenite and magnetite (Buddington and Lindsley, 1964). In the ocean crust, this is most often observed in basalts, dikes, or gabbros that were subjected either to high-temperature hydrothermal alteration (e.g., deep-dike rocks from Hess Deep) or to slow subsolidus cooling in the lower crust (gabbros from Hess Deep and the Southwest Indian Ridge). Indeed, during extensive hydrothermal alteration at greenschist or low-grade amphibolite facies temperatures, a common result is for all the magnetite to disappear (e.g., Shipboard Scientific Party, 1999; Natland, 2002), leaving only relict lamellae of ilmenite and no high-intensity magnetic minerals.
Site 1224 basalt thus presents a type of alteration of oxide minerals not previously seen, in that titanomagnetite is only partially destroyed with the pure magnetite component being partially removed from the mineral, leaving in the extreme a nearly pure ulvöspinel residuum. In effect, the Fe2O3-bearing component of the mineral is systematically and almost entirely removed. Ilmenite may experience a lesser but similar extraction of its Fe2O3-bearing component, hematite. As a result of this dissolution, iron, mainly in the oxidized state, must be added to the circulating solvent fluids, even at the very highest levels of the ocean crust. Evidently it is soon redeposited as iron oxyhydroxides and amorphous iron oxide in vein minerals and alteration haloes. Thus rather than viewing the sources of metals deposited at ridges to lie entirely within deep hydrothermal reaction zones near substantial magma bodies (e.g., Gillis et al., 2001), we must now consider that perhaps a considerable metal source results from low-temperature reactions occurring throughout the porous and fragmented upper ocean crust.
Glass Alteration
Because of hydration (sum of oxides is 84.6%–92.4%), the mobility of elements during formation of palagonite cannot be accurately assessed using absolute differences in composition between glass and its alteration product. When considered in terms of cation proportions on an anhydrous basis, glass altered to palagonite at pillow rims lost CaO and MgO and gained both total iron as FeOtot and K2O with respect to the nearly immobile oxides, TiO2, Al2O3, and SiO2. Na2O was enriched in some palagonites but depleted in others. The iron was probably provided by the same hydrothermal fluids that added iron oxyhydroxides to veins and alteration haloes in crystalline basalt and/or by low-temperature reactions (see previous paragraph). The K-rich composition of palagonite is ultimately derived from high-temperature nonoxidative alteration of abyssal tholeiites to mineral assemblages carrying chlorite and magnesian saponite deep in the section, since neither mineral allows K into its structure. The potassium is consequently released to the circulating hydrothermal fluids, and it later precipitates near the surface during oxidative formation of K- and Fe-smectite. Loss of CaO in palagonite occurred during transformation of glass to such clay minerals very near the seafloor, in a manner possibly analogous to loss of CaO during basalt alteration lower in the crust. The CaO lost in rocks at any depth plausibly contributes to the formation of late-stage veins of calcite and aragonite in these basalts.
Along with analyses of fresh glass, the compositions of black incipient spherulitic material and of orange palagonite replacing glass were obtained from the same thin sections (Table T5). The spherulitic material differs only slightly from fresh glass in having slightly higher MgO or Al2O3 and, in two cases, lower Na2O. Experimental studies and high-resolution microscopy show that spherulites in abyssal tholeiites are usually made up of extremely tiny needles, fibers, or dendrites of silicate minerals (e.g., Kirkpatrick, 1979). The bulk of the material in an individual spherulite must still be glass, and a defocused electron-probe beam will therefore necessarily intersect some combination of glass and, in these rocks, either plagioclase needles or clinopyroxene dendrites. The small departures in MgO and Al2O3 contents of spherulites from the adjacent glass indicate slight differences in proportions of minerals. Low Na2O contents in two spherulites may indicate that the glass fraction of the spherulite is altered to palagonite.
A zeolite composition reported in Table T6 (Sample 200-1224F-6R-1, 29–34 cm) is Na- and K-rich aluminosilicates resembling phillipsite. This phase, commonly found in palagonitized submarine vitric tuffs, is generally thought to incorporate alkalis, alumina, and silica derived from formation of palagonite. However, since Al2O3 and SiO2 in Site 1224 palagonites behave mostly as immobile oxides, this origin can be ruled out.

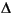 NNO). Most titanomagnetite, however, was strongly affected by low-temperature reaction with hydrothermal fluids, losing much of its magnetite (Mt) component to dissolution while retaining ulvöspinel. This may partly have provided the oxidized iron that is in the iron oxyhydroxide veins and adjacent alteration haloes.
NNO). Most titanomagnetite, however, was strongly affected by low-temperature reaction with hydrothermal fluids, losing much of its magnetite (Mt) component to dissolution while retaining ulvöspinel. This may partly have provided the oxidized iron that is in the iron oxyhydroxide veins and adjacent alteration haloes.