INTRODUCTION
Microbiological studies of the marine deep subsurface biosphere require methods to retrieve sediment samples without contamination by microorganisms from surface sediments or seawater or methods to quantify the extent of contamination. The ubiquitous contaminating medium is surface seawater used as a drilling fluid, which contains ~0.1 x 109 to 1 x 109 prokaryotes/L (Smith et al., 2000a). Here, we describe the further development and application of two methods to quantify the extent of seawater intrusion during drilling operations in deeply buried sediments. The concentration of a water-soluble chemical tracer was used as an indirect measure for the maximally possible entrainment of bacterial cells, and fluorescent microspheres were deployed as prokaryotic cell mimics to reveal the extent to which prokaryote-sized particles may have penetrated a sediment sample.
Characteristics
Perfluorocarbon tracers (PFTs) have been widely used in land-based drilling applications (Russell et al., 1992; McKinley and Colwell, 1996) because they are chemically inert and can be detected with high sensitivity. Perfluoromethylcyclohexane (C6F11CF3) was used as the chemical tracer to monitor potential seawater contamination of sediment and rock samples on the JOIDES Resolution (Smith et al., 2000a, 2000b). This perfluorocarbon compound (Aldrich 30293-7) has a molecular weight of 350.05 g, a boiling point of 76°C, and a density of 1.76 g/mL. Its solubility is ~2 mg/L in water and 104 mg/L in methanol (Colwell et al, 1992). The low solubility in water facilitates gas-phase partitioning and quantitative headspace analysis.
Delivery
Perfluorocarbon tracer was continuously fed into the stream of drilling fluid using a single-piston high-performance liquid chromatography pump (Alltech model 301). The tracer was delivered from a polypropylene carboy into the drilling fluid stream through a valve on the low-pressure side of the mud charge pump. The rate of the tracer injection was adjusted to maintain a final concentration of ~1 mg/L in the drilling fluid, using shipboard rig instrumentation software to control pumping rates (Smith et al., 2000a).
Sampling
Concentrations of PFT were measured in all sections used for microbiological studies. After core retrieval and the cutting of the 9.5-m core into six 1.5-m sections, samples for PFT measurement were immediately taken from the section ends adjacent to the section or sections that had been selected for microbiological analysis. Using sterile 5-mL syringes with the Luer lock end cut off, subcores of generally 3- to 4-cm3 volume were taken from the center of the sediment core and from the periphery of the sediment core at the core liner. In this way, parallel data sets were collected to show the extent of contamination in the periphery of the sediment core along the core liner and in the center of the core (Smith et al., 2000b). To increase the sensitivity of PFT detection, the sampling protocol was adjusted midcruise by taking two instead of one 5-mL syringe sample at the center of the core (Sites 1229-1231). Since the freshly cut sediment surface was potentially contaminated by PFT smear from the core liner, the sediment surface layer at the sampling spots was scraped away using a clean scalpel before syringe sampling. Special care was taken not to touch the syringe with hands or gloves that had come into contact with the core liner surface, which is heavily contaminated with drilling fluid and PFT tracer. The samples were placed in preweighed 20-mL headspace vials (Agilent) and were sealed with gas-tight stoppers. Liquid samples of 5 mL were taken from 25% sediment slurries prepared anaerobically for microbiological incubations and cultivations.
For positive qualitative controls of PFT delivery, a few drops of water from the outer core liner or from supernatant water on top of the first section of a core were collected in a headspace vial, then sealed and analyzed like the sediment samples.
Detection, Calibration, Sensitivity, and Background Controls
The conditions used on the gas chromatograph (GC) were somewhat different than those previously used by Smith et al. (2000a). We used a HP-PLOT/AL203 "S" deactivated column with film thickness = 50 µm, length = 15 m, phase ratio = 12, and column ID = 0.25 µm. The inlet temperature was 180°C with an inlet pressure of 10 psi. The detector temperature was 250°C. The column temperature was 100°C for 3.5 min and then ramped up at 50°C/min until it reached 200°C. The PFT peak eluted at 5.1 min. We used a 1-mL injection. Larger injections were found to result in loss of material. A calibration curve is shown in Figure F1.
The PFT detection limit reported for Leg 201 sites was not set as a lower limit of the ability to detect PFT by the GC but as a lower limit of the ability to confidently assess the presence of PFT in real samples, given the uncertainty inherent in subtracting background levels of PFT and the reliability of the integration of small GC peaks. Therefore, the detection limits for PFT at Sites 1295 and 1226 were in the range of 0.02 ng PFT (2 x 10-11 g)/g sediment or 0.02 µL potential seawater contamination/g sediment. After doubling the amount of sediment in a headspace vial during sampling at Sites 1229-1231, the detection limit was 0.01 µL seawater/g sediment. The laboratory air sometimes contained considerable PFT levels above background (up to 9 x 10-12 g PFT/mL air) (data for Site 1226), which would lead to false positive PFT concentrations for samples capped in the laboratory if no background correction was made. Very high PFT levels were found in the air of the cold room where the microbiology core sections were subsampled and processed (1.8 x 10-10 g PFT/mL air) (data for Site 1226). The PFT is almost certainly introduced on the core liner of the sections that are brought into the cold room directly from the catwalk. To avoid background problems, fresh samples were either carried to the catwalk for capping in the open air that had repeatedly tested PFT-negative or capped inside with a background PFT correction subtracted from the observed PFT concentration.
Sample Analysis
The headspace vials containing the sediment were heated for 5-10 min in an 80°C oven to evaporate and release the tracer from the sediment. Next, clean nitrogen gas was injected onto the GC column to make sure that no PFT peak resulted from residual PFT in the syringe or in the GC. After a clean run was achieved, the sample was injected using the same baked syringe. At the time of the injection, the syringe was also still hot so that PFT would not condense out before injection. Also, for best results, background air samples needed to be taken regularly from the same location used for capping headspace vials (ideally on the catwalk when no core is present). Finally, cleaning PFT out of used syringes was critical. We found it was best to use a large Hamilton syringe (10 mL) that could be flushed several times with air and then have the plunger removed for baking. Smaller syringes required a methanol wash to remove PFT and then had to be baked for a longer time to remove the methanol in order to avoid having an interfering GC peak. It was also found that cores with high levels of sulfide resulted in GC traces with small air peaks, presumably from the sulfide scrubbing oxygen from the headspace vial. Therefore, for cores rich in sulfide, the air peak cannot be used to normalize various GC runs.
In order to determine the PFT concentration from each sample, raw GC peak areas had to be corrected for injection size and headspace volume. After GC measurements, the vials were weighed to determine the amount of core material in each sample. The headspace of each vial used to calculate the PFT concentration was based on the total headspace (20 mL) minus the volume attributed to sediment calculated from the weight of the sediment divided by its bulk density. The calculation for liters of drill fluid per gram of sediment was as follows:
-
g PFT injected = (PFTs - PFTb) x CF, (1)
where
-
PFTs = PTF peak detected from sediment sample,
-
PFTb = PFT peak detected from the background, and
-
CF = factor from calibration curve.
-
g PFT/g sediment = [(g PFT injected) (V - W/
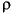 )/I)]/W, (2)
)/I)]/W, (2)
where
-
V = volume of headspace vial (mL),
-
W = the weight of the sediment sample (g),
-
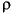 = the density of the sediment sample (g/mL), and
= the density of the sediment sample (g/mL), and
-
I = GC injection size (1 mL for these experiments).
In order to estimate the potential for seawater contamination in each sample, the concentration of PFT in each sample was converted to potential seawater contamination based on the 1 mg/L concentration of PFT in the seawater drilling fluid. Therefore, each nanogram of PFT detected in the sediment sample potentially represents 1 mL of seawater drilling fluid of contamination.
Characteristics
Fluorescent microspheres of a similar size (0.5 µm) to the indigenous microorganisms (0.2-1.3 µm) have been used previously in land-based drilling operations to assess dispersal and transport of these prokaryote cell mimics (Harvey et al., 1989). Yellow-green fluorescent microspheres (Fluoresbrite carboxylate microspheres; Polysciences Inc. 15700) with a diameter of 0.52 (±0.01) µm were used as a particulate tracer on the JOIDES Resolution. These microspheres are highly fluorescent (458-nm excitation; 540-nm emission) and appear bright green when observed by epifluorescence microscopy through a blue filter set (Zeiss filter set 09 or 10) (Smith et al., 2000a).
Delivery and Sample Collection
The desired concentration of microspheres at the point of drilling is ~1010 spheres/mL (Smith et al., 2000a). Microspheres were deployed in Whirl-pak bags containing 20 mL of microsphere suspension in deionized water (2 x 1011 microspheres/20 mL bag). Two different deployment procedures were used. Initially, the Whirl-paks were attached to one side of the core catcher (Sites 1225-1228); however, soft sediment cores could repeatedly slide past the attached bag without bursting it, with no concomitant release of microspheres. As a modification, the bag was filled with twice the volume of microsphere suspension (2 x 1011 microspheres/40 mL bag), and the attachment geometry in the core catcher was altered (Fig. F2). The bag was then heat-sealed such that extra plastic, not filled with beads, was left at each end. By attaching the loose plastic ends using Kevlar cord, the bag was wedged into a shim above the core catcher and stretched across the throat of the core barrel. Sediment cores were consequently forced to burst through the bead bag when a core was taken (Sites 1228-1231).
Microspheres were specifically deployed on cores that recovered sediment samples for microbiological cultivation and slurry preparation. For each slurry sample, three subsamples were analyzed: (1) a sample of the slurry, (2) a slurry sample diluted tenfold in 2% formaldehyde (also used for direct prokaryote counts), and (3) a scraping from the outer surface of the core to confirm deployment of beads.
Sample Collection and Counting Procedure
The sediment sample (5 mL of sediment or 10 mL of 25% sediment slurry) was mixed with an equal volume of saturated sodium chloride solution. The solution was centrifuged (Marathon 10K, 5 min, 2800 x g), and the supernatant was filtered onto black polycarbonate filters (0.2-µm pore size). Any fluorescent microspheres were counted under ultraviolet (UV) light, and data were reported as numbers of microspheres per gram of sediment.

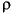 )/I)]/W, (2)
)/I)]/W, (2)