-
(dnCH4/dt) = 4
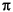 r2QCH4, (A1)
r2QCH4, (A1)
The growth rate of a bubble, after nucleation, is predominantly controlled by the mass flux of CH4 to the bubble. The change in mass within the bubble can be written as
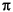 r2QCH4, (A1)
r2QCH4, (A1)
where QCH4 is the CH4 flux to the bubble per unit time. QCH4 depends on the diffusivity of CH4 in the pore fluid, DCH4, and its solubility, KHCH4, according to
 /4)], (A2)
/4)], (A2)
where
 = surface tension.
= surface tension.
The Sherwood number (Clift et al., 1978) accounts for increased mass flux to the bubble by reduction of the diffusive boundary layer around the bubble in the presence of flow; hence, when Sh = 1, the bubble growth is controlled by diffusion, and Sh > 1 can be assumed if the fluid is mixing during the ebullition process, as we expect. A characteristic timescale for the growth of a bubble of maximum radius, rmax, just before leaving the sediment into the headspace is, then,
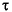 growth = (n/4
growth = (n/4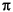 r2QCH4) =
r2QCH4) =
 /rmax)]}). (A3)
/rmax)]}). (A3)
Because CH4 controls the total pressure of the bubble, the bubble will scavenge trace gases (like N2 and Ar) as it grows in a characteristic time scale:
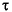 trace = (n/4
trace = (n/4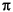 r2Qtrace) = (r2/3RTShDtraceKHtrace). (A4)
r2Qtrace) = (r2/3RTShDtraceKHtrace). (A4)
As the bubble grows, it will accumulate more quickly the more soluble and more diffusive trace gases from the pore fluid. The scavenging efficiency will depend on KHN2/KHCH4 (or KHAr/KHCH4) or, and to a lesser extent, on DN2/DCH4 (or DAr/DCH4) because for N2, Ar, and CH4, the greatest differences are in their solubility coefficients. As the ebullition process continues, the concentration of the trace gases in the pore fluid will decrease with time according to the ratio of 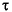 growth/
growth/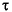 trace. Clearly, the headspace will initially accumulate the more soluble and more diffusive trace gases early on in the ebullition process.
trace. Clearly, the headspace will initially accumulate the more soluble and more diffusive trace gases early on in the ebullition process.