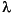 129 = 4.41 x 10–8/yr being the decay constant of 129I:
129 = 4.41 x 10–8/yr being the decay constant of 129I:
As a strongly biophilic element, iodine is commonly found heavily enriched in fluids associated with hydrocarbons, such as oil field brines (Moran et al., 1995) or coal-bed methane reservoirs (Snyder et al., 2003). Concentrations are particularly high in pore water associated with gas hydrates, where iodine is enriched typically by factors of 500 or more compared to seawater (Martin et al., 1993; Egeberg and Dickens, 1999; Fehn et al., 2003). The strong association between iodine and methane is not restricted to gas hydrate sites but is also observed in other methane-rich fluids in forearc settings (Muramatsu et al., 2001) or coal-bed methane reservoirs (Snyder et al., 2003). This association strongly suggests that these two compounds are derived from the same organic source and that the iodine system can be used to identify the formations responsible for the release of these two compounds. Accordingly, the iodine isotopic system has been used recently for the determination of source formations of hydrocarbons in a variety of settings, including investigations of gas hydrate occurrences at Blake Ridge (Fehn et al., 2000), Nankai Trough (Fehn et al., 2003), and the Peru margin (Fehn et al., in press).
Iodine has one stable isotope, 127I, and one long-lived radioisotope, 129I. 129I is a member of a group commonly called cosmogenic isotopes because they are produced mostly or partially by the interaction of cosmic rays with atoms of the atmosphere. It has the longest half-life (T1/2 = 15.7 Ma) of the radioisotopes in this group and is produced by the spallation of Xe isotopes in the atmosphere and by spontaneous fission of 238U in the crust. Both of these processes contribute similar amounts of natural 129I to surface reservoirs (Fabryka-Martin et al., 1985). Iodine moves quickly through most surface reservoirs, and natural iodine is isotopically homogeneous at the surface of the Earth, including oceans, shallow sediments, and the biosphere. The exchange between marine and terrigenous sources is sufficiently rapid to have an isotopically well mixed signal in surface reservoirs at the resolution available for natural 129I concentrations. Preanthropogenic 129I/I ratios therefore provide identical age dependence for marine and terrigenous sources, although terrigenous sources typically have lower iodine concentrations. Anthropogenic 129I is a third component present in surface reservoirs and is related to releases from weapons tests and reprocessing plants. The widespread occurrence of anthropogenic 129I in rivers and lakes throughout the world is evidence for the high mobility of this element on a global scale (Santschi and Schwehr, 2004; Snyder and Fehn, 2004). Although the current isotopic equilibrium in surface reservoirs has been substantially disturbed because of this contribution (e.g. Schink et al., 1995; Moran et al., 1999; Oktay et al., 2001), the influence of anthropogenic 129I is mostly restricted to the zone of bioturbation (Fehn et al., 1986; Moran et al., 1998), with minor penetration possible beyond that zone as observed in the Gulf of Mexico (Oktay et al., 2000).
Because iodine has only one stable isotope, 127I, its isotopic composition is reported as 129I/I. The long residence time (300,000 yr) of iodine in the oceans (Broecker and Peng, 1982) provides a homogeneous isotopic signal in the marine system. Recent sediments below the zone of bioturbation (i.e., without the presence of anthropogenic I) have 129I/I ratios of Ri = (1500 ± 150) x 10–15 (Fehn et al., 1986; Moran et al., 1998). Ratios supporting this value were also found in determinations of preanthropogenic seawater and algae (e.g., Schink et al., 1995; Cooper et al., 1998; Y. Muramatsu, unpubl. data, 2002). This ratio, which is used as starting value for 129I-based age calculations, is two orders of magnitude above the detection limit of accelerator mass spectrometry (AMS), the method of choice for the detection of cosmogenic radioisotopes. The half-life of 129I, together with the observed input ratios and the detection limit for 129I/I (2 x 10–14) (Sharma et al., 2000), allows applications of this dating method within a range of ~80 m.y. The calculation of iodine ages tmin from a measured ratio Rm uses the following equation, with 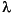 129 = 4.41 x 10–8/yr being the decay constant of 129I:
129 = 4.41 x 10–8/yr being the decay constant of 129I:
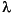 129). (1)
129). (1)
Depending on the geologic situation, a correction for the contribution of fissiogenic 129I has to be taken into account. The addition of fissiogenic 129I is a function of age and uranium concentration of the host formation and the probability of transport from host formation into the fluids. Although the addition of fissiogenic 129I presents an uncertainty for the ages calculated, the potential correction in the age range found here is probably <5 m.y. in the oldest samples found. Because the presence of either fissiogenic or anthropogenic 129I would have raised the measured ratio, ages calculated from equation 1 are minimum ages. The presence of anthropogenic 129I typically increases the ratio beyond the preanthropogenic input value and is an unlikely presence in marine sediments beyond the layer of bioturbation (Fehn et al., 1986; Moran et al., 1998), but it can play a role in samples taken from open systems on land.
Concentrations of iodine in surface fluids are generally low, 0.4 µM (55 ppb) in seawater (Geochemical Reference Model, earthref.org/GERM) and <0.02 µM in freshwater (e.g., Moran et al., 1999), depending in part on the presence of organo-iodine compounds (Schwehr and Santschi, 2004). Associated with organic material, iodine can be considerably enriched: typical concentrations in oil field brines are ~80 µM (e.g. Moran et al., 1995), and concentrations as high as 2 mM were found in pore water associated with gas hydrates (Egeberg and Dickens, 1999) and in methane-rich cold seeps of forearc areas (Muramatsu et al., 2001). Molecular diffusion coefficients depend on the speciation of iodine and vary between 1.08 x 10–5 cm2/s for iodate and 2.05 x 10–5 cm2/s for iodide, bracketing the value for methane (1.49 x 10–5 cm2/s), but are much lower for organo-iodine compounds (all values for 25°C are from Linde, 2004). Because the predominant iodine species in deep, methane-rich fluids is iodide (Muramatsu et al., 2001), the similarity of diffusion coefficients suggests that methane and iodine travel together in fluids. This statement applies as long as methane does not convert into the gas phase, a situation that might occur in and below the gas hydrate stability zone (Torres et al., 2004).
The investigation of iodine concentrations and isotope ratios is accompanied by a study of bromine and chloride concentrations. Of the three halogens investigated here, iodine has the strongest biophilic character, chlorine has little association with organic material, and the association of bromine with organic matter falls in between those two characteristics. Concentrations of chlorine in pore water associated with gas hydrates typically are at or somewhat below that of seawater, often accompanied by freshening in the layer just above the bottom-simulating reflector (BSR). This observation is commonly related to the release of water due to the dissolution of gas hydrate crystals and is used for the quantification of the amount of gas hydrates at a given location (e.g., Ussler and Paull, 2001; Tomaru et al., 2004). In several gas hydrate locations, chlorine concentrations decrease consistently with depth, suggesting an influx of deep waters with Cl concentrations below that of seawater (Ussler and Paull, 2001; Hesse, 2003). Chlorine has also a cosmogenic isotope (36Cl; T1/2 = 0.3 Ma), but because of the high concentration of "dead" Cl in seawater the ratio, 36Cl/Cl is below the detection limit of AMS. Chlorine also has two stable isotopes, 35Cl and 37Cl, and their ratio has recently been used for the detection of sources of fluids in gas hydrate settings (e.g., Hesse, 2003). Studies of this kind have not been carried out for Hydrate Ridge so far.
Bromine concentrations follow a pattern similar to that of iodine but with considerably lower enrichment factors (e.g., Egeberg and Dickens, 1999). Element ratios are useful for the determination of the origin of fluids (e.g., Frape et al., 2004) and of the organic source material (Muramatsu et al., 2001), and they therefore complement the observations based on concentrations or isotope ratios.