INORGANIC GEOCHEMISTRY
Pore Fluid Sampling and Chemical Analyses
Shipboard pore fluid analyses were performed on fluids squeezed from 15- to 45-cm-long whole-round sections that were cut and capped immediately after the core retrieval on deck. One to two whole-round samples per core were obtained from the section of the core with little to no apparent drilling disturbance. The sediment was immediately removed from the core liner, and the outer layer, which mostly consists of sediments permeated by drill water (surface seawater) spiked with perfluoro(methylcyclohexane) (C7F13) and fluorescent latex microspheres, was carefully trimmed to remove any potential contaminants. The cleaned pieces of sediment were placed into a titanium squeezer (modified after Manheim and Sayles, 1974) between one or two Whatman number 1 filters that were previously rinsed in high-purity water to remove processing acids. The hydraulic press was operated up to a maximum pressure of ~30,000 psi (205 MPa). The sediment sample remained under pressure until most of the squeezable water was removed. Pore fluid was collected into prewashed 10- to 50-cm3 plastic syringes and filtered through disposable 0.45-µm Gelman polysulfone filters. Samples were stored in acid-cleaned plastic vials pending shipboard analyses. Aliquots for future shore-based isotopic analyses were placed in glass ampoules and heat sealed, and aliquots for future trace metal and elemental analyses were placed in three acid-washed plastic vials and acidified to 10% with Optima-grade nitric acid. Samples for future dissolved inorganic carbon analyses were poisoned with a saturated mercuric chloride solution, and sulfide was precipitated with a 50% CdNO3 solution.
Pore water samples were routinely analyzed for salinity and total dissolved solids with a Goldberg optical handheld refractometer (Reichert) and for pH and alkalinity by Gran titration with a Brinkmann Instruments pH electrode and a Metrohm autotitrator. Chloride concentration was determined by titration with AgNO3. Calcium and magnesium concentrations were analyzed by ethylene-bis(oxyethylenitrilo)tetraacetic acid and disodium ethylenediamine tetraacetic acid titrations, respectively. Silica and ammonium determinations were carried out by colorimetry using a Milton Roy Spectronic 301 spectrophotometer and a Milton Roy Mr. Sipper introduction system (Gieskes et al., 1991). Pore fluids were not analyzed for phosphate because of the low (~1 µM) and constant concentration observed during Leg 170 within the intervals cored during Leg 205.
International Association of Physical Sciences Organizations (IAPSO) standard seawater was used for calibrating all techniques. The reproducibility of these analyses, expressed as percent precision calculated from multiple determinations of IAPSO standard seawater or of a standard are alkalinity,  3%; chloride,
3%; chloride,  0.15%; calcium,
0.15%; calcium,  1%; magnesium,
1%; magnesium,  1%; silica,
1%; silica,  2%; and ammonium, 4%; and the accuracy for silica is
2%; and ammonium, 4%; and the accuracy for silica is  4%. At all sites, sodium was determined using charge balance calculations, where
4%. At all sites, sodium was determined using charge balance calculations, where 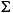 (cation charge) =
(cation charge) = 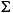 (anion charge), as well as by ICP-AES.
(anion charge), as well as by ICP-AES.
Sulfate was analyzed by ion chromatography (ICr) using the Dionex DX-120. The reproducibility of the sulfate analyses, expressed as percent precision calculated from multiple determinations of IAPSO standard seawater or of a standard is  2%. Potassium, calcium, and magnesium concentrations were not determined by ICr because of an unidentified problem that persisted even after exchanging the cation column and the suppressor. These elements were analyzed by ICP-AES, which provides better precision than the ICr.
2%. Potassium, calcium, and magnesium concentrations were not determined by ICr because of an unidentified problem that persisted even after exchanging the cation column and the suppressor. These elements were analyzed by ICP-AES, which provides better precision than the ICr.
The major cations and the minor elements: lithium, boron, strontium, barium, rubidium, manganese, iron, and silica were determined by using the Jobin-Yvon Ultrace ICP-AES following the general procedure outlined by Murray et al. (2000) and modified by M. Delaney. The shipboard "master" ICP standard (Murray et al., 2000; modified by M. Delaney) was expanded so that Si concentrations could be determined. In addition, a special external standard was prepared for rubidium because of the element's high detection limit (~50 ppm) on the ICP-AES. In preparation for analysis by ICP-AES, aliquots of interstitial waters were acidified with nitric acid (HNO3) and diluted tenfold (2.25% HNO3 and 9 ppm Y) with nanopure water for the minor elements and diluted fifty times (2.25% HNO3 and 9 ppm Y) for the major elements. Analytical blanks were prepared identically by analyzing nanopure water, which was acidified to matrix levels, and Y was added to match the samples. A detailed flow chart for preparation of pore fluid samples for ICP-AES analyses is available in Figure F16. At all sites, sodium was also determined using charge balance calculations.
The reproducibility of the major and minor element analyses, expressed as percent precision calculated from multiple determinations of IAPSO standard seawater or of standards and samples are sodium,  0.1%; magnesium,
0.1%; magnesium,  0.2%; potassium,
0.2%; potassium,  0.4%; calcium,
0.4%; calcium,  0.3%; boron, lithium, and barium,
0.3%; boron, lithium, and barium,  2%; iron and manganese,
2%; iron and manganese,  3%; Si and Sr,
3%; Si and Sr,  1%. Accuracies of the ICP-AES analyses for these elements, as determined by standards run as unknowns, are sodium, <0.5%; magnesium, <1%; potassium, <4%; calcium, <0.5%; boron, ~8%; barium, <3%; iron, <5%; lithium, ~4%; manganese, ~6%; silica, <4%; and strontium, <6%. Rubidium concentrations were lower than the detection limit of this ICP-AES.
1%. Accuracies of the ICP-AES analyses for these elements, as determined by standards run as unknowns, are sodium, <0.5%; magnesium, <1%; potassium, <4%; calcium, <0.5%; boron, ~8%; barium, <3%; iron, <5%; lithium, ~4%; manganese, ~6%; silica, <4%; and strontium, <6%. Rubidium concentrations were lower than the detection limit of this ICP-AES.
Sediment Sampling and Chemical Analyses
Bulk sediment samples were analyzed routinely during Leg 205. Samples for bulk sediments were taken from interstitial water squeezed cakes and from select sediment horizons chosen by the sedimentologists. Bulk samples were prepared according to the method of Murray et al. (2000). Samples were first freeze-dried and powdered. Samples and standards (0.1 g) were mixed with LiBO2 flux (0.4 g). Analytical blanks were prepared using 0.4-g LiBO2 flux to ensure matrix matching. A solution of 0.172-mM LiBr wetting agent (10 µL) was added to the samples, standards, and blanks. This mixture was fused for 3 min at 1025°C in an NT-2100 Bead Sampler prior to dissolution in 50 mL of 10% HNO3. A 5-mL aliquot of the resulting solutions was filtered (0.45 µm) and diluted with 35 mL of 10% HNO3, resulting in a 4000-fold dilution of the original powders (refer to Fig. F17). Na, Mg, K, Ca, Sr, Ba, Fe, Mn, Cu, Al, Ti, Si, P, Cr, Zr, Y, and V concentrations were determined using a Jobin-Yvon Ultrace ICP-AES using a Type-C Meinhard concentric nebulizer following the general procedure outlined by Murray et al. (2000).
For these analyses, the certified standards SGR-1 (Green River shale), MAG-1 (marine mud), SCO-1 (Cody shale), NBS-1c (argillaceous limestone), NBS-99a (sodium plagioclase), BIR-1 (Icelandic basalt), and BHVO-2 (Hawaiian basalt) were used to develop a seven-point calibration curve for the major and most minor and trace elements. The precision and accuracy of SGR-1 were consistently inadequate; therefore, this standard was omitted from the calibration curve and subsequent batches of analyses. NBS-1c and NBS-99a do not have certified analyses of the minor and trace elements; Washington University minor and trace element chemistry for the NBS-1c was used instead. These standards were chosen because they are most representative of the lithologies present at Sites 1253 and 1254 as determined by careful inspection of X-ray fluorescence major, minor, and trace element data generated during Leg 170. MAG-1 was chosen as a drift standard because it has higher concentrations of elements analyzed during Leg 205 than the other four standards. One drift standard was run for every four samples (two standards followed by two samples), and one standard was run as an unknown every five samples. The drift and standard samples were also analyzed at the beginning and end of each ICP-AES batch of analyses. Each sample was run six times, and two LiBO2 flux blanks were run per batch of analyses.
The reproducibility of these analyses, expressed as percent precision calculated from multiple determinations of standards and samples are Al and Sr, <2%; Na, Mg, V, and Si, <3%; Ba, Ca, and Mn, <4%; Fe and K, <5%; Ti and Cr, <6%; Zr, <10%. Y reproducibility, except for three sediment samples, was <8%. The reproducibility for P was low (<25%), and Nb, Ni, Cu, and Zn reproducibilities and/or accuracies were deemed unacceptable (25%).

 3%; chloride,
3%; chloride,  0.15%; calcium,
0.15%; calcium,  1%; magnesium,
1%; magnesium,  1%; silica,
1%; silica,  2%; and ammonium, 4%; and the accuracy for silica is
2%; and ammonium, 4%; and the accuracy for silica is  4%. At all sites, sodium was determined using charge balance calculations, where
4%. At all sites, sodium was determined using charge balance calculations, where 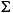 (cation charge) =
(cation charge) = 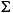 (anion charge), as well as by ICP-AES.
(anion charge), as well as by ICP-AES.