SAMPLE PREPARATION
The JOIDES Resolution flux fusion method is detailed in Murray et al. (2000), Quintin et al. (2002), and Wilson, Teagle, Acton, et al. (2003), and is only summarized here. The 28 bulk sediment samples (1 per core) were sampled as a split from the interstitial water squeeze cakes, freeze-dried, and powdered manually with an agate mortar and pestle. Each powdered sample was first ignited as part of the loss on ignition (LOI) analysis to release volatile phases and to fully oxidize all iron to ferric iron. Each ignited sample was mixed with LiBO2 flux in a 1:4 sample/flux ratio in a Pt-Au crucible coated with a LiBr wetting agent to avoid sticking and heated at 900°C in a NT-2100 Bead Sampler furnace for 3 min. Upon subsequent cooling, the now glass bead sample was dissolved in 50 mL of 10% HNO3 to yield a 1000-fold dilution. Once the glass bead was completely dissolved, the aqueous solution was filtered and further diluted 4000-fold for instrumental uptake.
At Boston University, we randomly selected 35 samples from the 100 sample shore-based set. We sampled from the same squeeze cake split that was used for the shipboard analyses. The general procedures were similar to those used during the cruise, with three exceptions. First, we did not perform an LOI analysis because it is not recommended for biogenic-rich sediments (Murray et al., 2000). The LOI comprises a significant portion of the total mass of the sample, and thus potentially introduces error in the total and often compromises the alkali results due to their inadvertent loss via volatilization. Considering that carbonate sediments are an important portion of the sample set, we bypassed the LOI analysis and thus maintained procedural consistency through all lithologies. Thus, when comparing the shipboard and shore-based results, we calculated all concentrations on an anhydrous basis. Second, on board the JOIDES Resolution, the use of Pt-Au crucibles required a wetting agent, whereas the use of graphite crucibles at Boston University does not. Third, the furnace temperature was increased to 1050°C and the fusing time increased from 3 to 12 min. The ovens used in the two laboratories are fundamentally different, with the JOIDES Resolution facility being a special unit with a rapid temperature rise whereas at Boston University we use a standard muffle furnace.
Shore-Based Acid Digestion
All 100 samples for the shore-based acid digestion sample set were acquired from the interstitial water squeeze cakes that were taken at a higher resolution (2 per core). Some of these samples were also splits of the shipboard samples. The objectives for the higher resolution sample set were to capture the complete chemical variability at Site 1256 and to better characterize the entire sediment sequence, and the scientific significance of these results will be presented elsewhere. Only 10 items (samples, standards, blanks, etc.) can be processed per batch when using the microwave system; therefore, 12 batches were required to digest all samples, with multiple replicates, standards, and blanks distributed between the 12 batches.
Approximately 50 mg of freeze-dried, powdered sample was placed into a microwave digestion vessel that contained a mixture of HNO3, HCl, and HF (6:2:2 mL, respectively). The vessel was then tightly sealed and placed into a Milestone Ethos-Plus microwave system (Milestone Inc., Shelton Connecticut, USA). The first three step-wise temperature settings consisted of ramping to 160°C over 12 min, then to 210°C over 8 min, and finally holding constant at 210°C for 30 min. The vessels vented for 30 min and cooled to <50°C, whereby they were opened and 0.5 mL of 30% hydrogen peroxide (H2O2) and 10 mL of 5% boric acid (H3BO3) were added immediately. For samples with a higher organic content (approximately >0.5 wt% Corg), 1 mL of H2O2 was used. Vessels were immediately resealed and placed back into the microwave for a second digestion. This second digestion consisted of a ramp time of 8 min to 160°C, and the temperature was held constant at 160°C for an additional 7 min. The vessels were again vented for 30 min and cooled to 50°C prior to opening. Each sample was then transferred to a preweighed bottle and brought to a total of 50 g with deionized water (Milli-Q, 18 M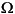 ) for a 1000-fold dilution. We selected a 1000-fold dilution factor to ensure a better signal-to-noise ratio for the lower concentration trace elements in carbonates while also maintaining a low amount of total dissolved solids (TDS). To minimize the potential for clogging of the nebulizer on the ICP-AES with this 1.1% TDS solution, we used a Teflon nebulizer and spray chamber. We recommend a total 6000-fold dilution factor for the more commonly used Meinhardt concentric glass nebulizers. However, use of the Teflon system we employed also provides the additional benefit of preventing enhanced consumption of glass nebulizers and spray chambers.
) for a 1000-fold dilution. We selected a 1000-fold dilution factor to ensure a better signal-to-noise ratio for the lower concentration trace elements in carbonates while also maintaining a low amount of total dissolved solids (TDS). To minimize the potential for clogging of the nebulizer on the ICP-AES with this 1.1% TDS solution, we used a Teflon nebulizer and spray chamber. We recommend a total 6000-fold dilution factor for the more commonly used Meinhardt concentric glass nebulizers. However, use of the Teflon system we employed also provides the additional benefit of preventing enhanced consumption of glass nebulizers and spray chambers.

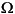 ) for a 1000-fold dilution. We selected a 1000-fold dilution factor to ensure a better signal-to-noise ratio for the lower concentration trace elements in carbonates while also maintaining a low amount of total dissolved solids (TDS). To minimize the potential for clogging of the nebulizer on the ICP-AES with this 1.1% TDS solution, we used a Teflon nebulizer and spray chamber. We recommend a total 6000-fold dilution factor for the more commonly used Meinhardt concentric glass nebulizers. However, use of the Teflon system we employed also provides the additional benefit of preventing enhanced consumption of glass nebulizers and spray chambers.
) for a 1000-fold dilution. We selected a 1000-fold dilution factor to ensure a better signal-to-noise ratio for the lower concentration trace elements in carbonates while also maintaining a low amount of total dissolved solids (TDS). To minimize the potential for clogging of the nebulizer on the ICP-AES with this 1.1% TDS solution, we used a Teflon nebulizer and spray chamber. We recommend a total 6000-fold dilution factor for the more commonly used Meinhardt concentric glass nebulizers. However, use of the Teflon system we employed also provides the additional benefit of preventing enhanced consumption of glass nebulizers and spray chambers.