
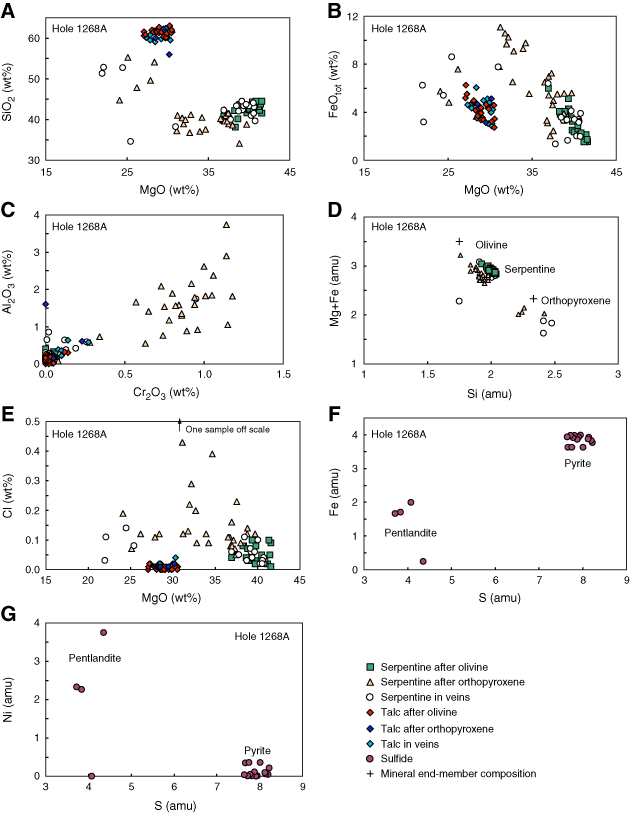
Figure F4. Plots of chemical variations of secondary phases (serpentine, talc, and sulfide) in Hole 1268A. A. SiO2 vs. MgO of serpentine and talc after olivine and orthopyroxene, and filling veins. B. FeO vs. MgO of serpentine and talc forming pseudomorphs after olivine and orthopyroxene, and filling veins. C. Al2O3 vs. Cr2O3 of serpentine and talc after olivine and orthopyroxene, and filling veins. D. Cl vs. MgO of serpentine and talc after olivine and orthopyroxene, and filling veins. E. Cation proportions Mg + Fe vs. Si of serpentine and talc forming pseudomorphs after olivine and orthopyroxene, and filling veins. Mineral end-members are shown (amu = atomic mass unit). F. Cation proportions Fe vs. S of sulfides (pentlandite and pyrite). G. Cation proportions Ni vs. S of sulfides (pentlandite and pyrite).

![]()