-
2CH2O + SO42–
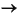 H2S + 2HCO3–
H2S + 2HCO3–
In most cases where ODP is coring, H2S forms as a byproduct of the degradation of organic matter. Because sulfate is plentiful in the ocean, sulfate reduction and, hence, hydrogen sulfide production is common in marine sediments.
Specifically, H2S forms during sulfate reduction via the generalized formula
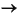 H2S + 2HCO3–
H2S + 2HCO3–
where
Despite their common presence in marine sediments, H2S concentrations do not usually reach very high levels. Relatively low concentrations of H2S are maintained primarily because either the reactants (organic matter and/or sulfate) may be limited or iron combines with the sulfide to form iron sulfide minerals (e.g., pyrite).
The highest H2S concentrations encountered during Deep Sea Drilling Project (DSDP)/ODP operations were located in the carbonate-rich sediments of the south Australian margin (Leg 182; 150,000 ppm in a single core). High sedimentation rates, low iron content, and sulfate-rich pore fluids led to extensive production of H2S.
Typically, diffusion from overlying seawater is the main source of sulfate. However, on some continental margins brines may provide an additional source of sulfate for sulfate reduction and production of H2S.
In areas where overlying seawater is the main source of sulfate, H2S concentrations will eventually decrease with depth. Even when there is high organic matter content, sulfate eventually becomes depleted with depth. Thus, H2S may be encountered over several cores but, like sulfate, H2S should also decline with depth.
Typical environments where H2S may be present are terrigenous continental margins, carbonate-rich continental margins or carbonate banks, gas hydrate sediments, and hydrothermal systems.
H2S may be present in terrigenous continental margin environments, especially where coastal upwelling results in sediments with high organic matter content. Typically in this setting, the sedimentary components often provide plenty of available iron, resulting in the removal of H2S via iron sulfide precipitation.
Slopes of carbonate-rich continental margins or carbonate banks with high sedimentation rates may be a source of H2S. Although carbonate-rich sediments are typically not rich in organic matter, the slopes can be characterized by high sedimentation rates. High accumulation rates bury organic matter, creating an increased potential for organic matter availability for sulfate reduction. Carbonate-rich sediments also have low iron contents, so H2S is rarely quantitatively precipitated as iron sulfides.
Another potential point source of H2S on continental margins is gas hydrates (clathrates). Although most natural marine hydrates are dominated by methane, H2S-methane hydrates have been encountered (e.g., Cascadia margin, Legs 146 and 182). Incorporation of H2S into the hydrate structure shifts the hydrate stability field; hence, H2S-rich hydrates are found at shallower depths than methane hydrates.
H2S may be encountered in hydrothermal systems (e.g., massive sulfide deposits). Consequently, appropriate precautions should be in place when recovering such fluids or coring sediments whose pore fluids may contain a hydrothermal component.