-
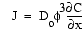 , (4)
, (4)
The flux of sulfate into the sediment from overlying seawater can be approximated from linear sulfate gradients using Fick's First Law:
where J is the flux, Do
is free-solution diffusion coefficient, ![]() is porosity, and
is porosity, and ![]() C/
C/![]() x
is the concentration gradient (e.g., Lerman, 1979). Given a mean sediment
porosity of 70% for the sulfate reduction zone (Paull, Matsumoto, Wallace, et
al., 1996), sediment temperatures of ~4║C (Ruppell, 1997), and a sulfate
diffusion coefficient of 5.8 x 10-6 cm s-1 (5║C, Li and
Gregory, 1974), the sulfate gradients from Sites 994, 995, and PC 11-8 predict
sulfate fluxes of (8.8, 8.2, and 18) x 10-4 mmol cm-2 yr-1,
respectively.
x
is the concentration gradient (e.g., Lerman, 1979). Given a mean sediment
porosity of 70% for the sulfate reduction zone (Paull, Matsumoto, Wallace, et
al., 1996), sediment temperatures of ~4║C (Ruppell, 1997), and a sulfate
diffusion coefficient of 5.8 x 10-6 cm s-1 (5║C, Li and
Gregory, 1974), the sulfate gradients from Sites 994, 995, and PC 11-8 predict
sulfate fluxes of (8.8, 8.2, and 18) x 10-4 mmol cm-2 yr-1,
respectively.
An established approach of
assessing the importance of AMO is to algebraically account for the carbon
isotopic contributions of each component comprising the ![]() CO2
pool (Blair and Aller, 1995). The
CO2
pool (Blair and Aller, 1995). The ![]() CO2
sources at the sulfate-methane interface include that from overlying seawater
trapped within sediments during burial (sw), that derived from sedimentary
organic matter during remineralization (om), and that produced by anaerobic
methane oxidation (amo). In a closed system, the carbon isotopic composition of
the pool at the SMI is related to its components by:
CO2
sources at the sulfate-methane interface include that from overlying seawater
trapped within sediments during burial (sw), that derived from sedimentary
organic matter during remineralization (om), and that produced by anaerobic
methane oxidation (amo). In a closed system, the carbon isotopic composition of
the pool at the SMI is related to its components by:
where X is the
constituent's fraction of the total ![]() CO2
pool,
CO2
pool, ![]() 13C
is the carbon isotopic composition, and the subscripts sw, om, and amo refer to
CO2 donated from the sources listed above. For Site 995, the value of
Xsw is fixed at 0.139 (seawater
13C
is the carbon isotopic composition, and the subscripts sw, om, and amo refer to
CO2 donated from the sources listed above. For Site 995, the value of
Xsw is fixed at 0.139 (seawater ![]() CO2
concentration divided by
CO2
concentration divided by ![]() CO2
concentration at the SMI, or 2.3 mM/ 16.5 mM) with
CO2
concentration at the SMI, or 2.3 mM/ 16.5 mM) with ![]() 13Csw
equal to 0
13Csw
equal to 0![]() .
The isotopic value of sedimentary organic matter at the Blake Ridge averages -21
.
The isotopic value of sedimentary organic matter at the Blake Ridge averages -21![]() ▒ 3
▒ 3![]() PDB (N= 120; Brooks et al., 1983; Olsen, 1997), and this value is used for
PDB (N= 120; Brooks et al., 1983; Olsen, 1997), and this value is used for ![]() 13Com.
The isotopic composition of methane at the interface is -101
13Com.
The isotopic composition of methane at the interface is -101![]() PDB (Hoehler et al., Chap.
8, this volume), and this value is used for
PDB (Hoehler et al., Chap.
8, this volume), and this value is used for ![]() 13Camo
(assuming no fractionation during AMO). The proportions of Com and Camo
composing the remaining fraction of the
13Camo
(assuming no fractionation during AMO). The proportions of Com and Camo
composing the remaining fraction of the ![]() CO2
pool (1.0 - 0.139 = 0.861) are varied to produce the mixing line in Figure
4.
CO2
pool (1.0 - 0.139 = 0.861) are varied to produce the mixing line in Figure
4.
The mixing model estimates that ~24% of the carbon within the CO2 pool at the sulfate-methane interface is derived from methane (Fig. 4). This is only a crude estimate because the system is open (i.e., CO2 diffusion occurs), because carbonate mineral precipitation occurs (Rodriguez et al., Chap. 30, this volume), and because carbon fractionation occurs during AMO.
Modeling of methane concentrations provides an independent estimate of the amount of sulfate consumed by AMO. Although in situ methane concentrations of deep-water sediments are difficult to measure accurately (Paull et al., unpubl. data), methane concentrations less than the methane bubble saturation at surface temperature and pressure should reflect in situ concentrations because loss of methane through outgassing is unlikely. Hence, the methane concentration data (Table 3) are apparently suitable for modeling.
The method assumes steady state conditions so that the distribution and magnitude of reaction rates can be solved for by fitting observed concentration data and solving the diagenetic equation (Eq. 3) for Rx using numerical methods. A cubic spline fit to the methane concentrations measured in Site 995 sediments (Hoehler et al., Chap. 8, this volume) approximates these data well (Fig. 5A). The measured methane profile has inflections (see arrows Fig. 5A), accurately reflected in the cubic spline fit, that produce variations in the first and second derivatives. The model results identify the depths where methane consumption and methane production are expected, and indicates the magnitude of the reaction rates as a function of depth (Fig. 5B). Model results also show that methane consumption occurs over a 2-m interval (~20.5-22.5 mbsf), and that methane production occurs immediately below (Fig. 5B). The modeled AMO reaction rates show peak values approaching 5 ÁM yr-1 (0.005 Ámol cm-3 yr-1) just below 21 mbsf.
The integrated methane flux corresponds to the area under the first derivative of the cubic spline fit (not shown). The model predicts a total upward methane flux of 2.9 x 10-4 mmol cm-2 yr-1. At Site 995, the corresponding total sulfate flux, calculated using Fick's First Law, is 8.2 x 10-4 mmol cm-2 yr-1. Thus, approximately 35% of the total sulfate flux into the sediment is used to consume upwardly diffusing methane.
Model results from fitting
the methane concentration values agree well with concentration and isotopic
data. As expected, the slight inflection in the concentration trend within the
methanogenic zone (Fig. 5A)
causes the model to predict a shift from methane production to methane
consumption, and the large inflection in methane concentration at the SMI
coincides with peak methane oxidation rates. Furthermore, minimum ![]() 13C
13C![]() CO2
values are coincident with peak methane oxidation rates.
CO2
values are coincident with peak methane oxidation rates.