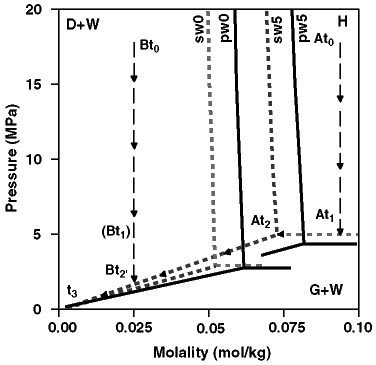
Figure 2. Concentration-pressure phase diagrams for pure CH4-pure water (pw) and pure CH4-seawater (sw) systems at constant temperatures of 0ºC and 5ºC (Handa, 1990; Dickens and Quinby-Hunt, 1994). There are three phases--CH4 gas (G), CH4 hydrate (H), and water with dissolved CH4 (W)--separated by three equilibrium curves. The intersection of the equilibrium curves exists for seawater at 0.052 mol/kg and 2.89 MPa at 0ºC, and 0.072 mol/kg and 4.95 MPa at 5ºC (Handa, 1990). Note that experimental data for equilibrium curves separating CH4-hydrate (H) and CH4 gas undersaturated water (W) are unavailable and that points defining these "partial saturation" curves are theoretical (Handa, 1990). Note also that gas and water will be in equilibrium with ice (I) in the case of pure water and CH4 at 0ºC (see Fig. 1). A core recovered at high pressure will change positions on this diagram as pressure is released through incremental gas volume loss over time. Path A is the expected change in position for a core with initial CH4 molality of 0.09 mol/kg maintained at constant temperature of 5ºC as pressure is decreased from initial pressure of 18 MPa at At0 to 4.95 MPa at At1 and At2, and to 0.10 MPa at t3. Path B is the expected change in position for a core with an initial CH4 molality of 0.025 mol/kg maintained at constant temperature of 5ºC as pressure is decreased from initial pressure of 18 MPa at Bt0 to 1.71 MPa at Bt2' and to 0.10 MPa at t3. Note that a core following Path A would contain gas hydrate and dissolved gas in seawater at initial conditions, whereas a core following Path B would contain only dissolved CH4 in seawater.
![]()