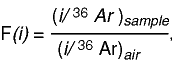
Shipboard results of gas dissociation experiments are presented in Table 2 and by Paull, Matsumoto, Wallace, et al. (1996). Gas volumes released from dissociation of hydrate specimens ranged between 183 and 1250 cm3 and were significantly larger than the volume of the chamber (23 cm3). All gas splits analyzed on ship contained in excess of 98.4% CH4 with the remainder consisting largely of CO2. Water volumes released from dissociation of hydrate specimens ranged between 3.3 and 9.1 cm3, and had Cl- concentrations between 21 and 294 mM. Volumetric gas/water ratios for samples after hydrate dissociation vary between 20 and 154. In general, volumetric gas/water ratios are proportional to gas volumes and inversely proportional to Cl- concentrations (Table 2).
Noble gas data is typically presented in terms of F(i) values. An F(i) value is the fractionation factor relative to atmospheric composition, and is defined as
where (i) is the concentration of an isotope of a noble gas. Concentrations of 36Ar and F(i) values (with uncertainties) for the most abundant stable isotope of He, Ne, Kr, and Xe are listed in Table 3 for the gas samples obtained on Leg 164. Relative to air, all samples are enriched in 4He and 132Xe (F[i] > 1), and depleted in 22Ne (F[i] < 1); most samples are enriched in 84Kr.
Helium isotope data is typically presented in terms of R/Ra values where R is the measured 3He/4He value in a sample, and Ra is the 3He/4He value in air. R/Ra values (with uncertainties) for the gas samples obtained on Leg 164 range between 0.11 and 1.01 (Table 3). The isotopic compositions of Ne, Ar, and Xe were isotopes were indistinguishable from air.