BASIC
INTERPRETATIONS
Two related questions
provided the impetus for this investigation: (1) is natural CH4
hydrate significantly enriched in Xe relative to the other noble gases, and (2)
can Xe in oceanic CH4 hydrate account for Xe "missing" from
the atmosphere? However, our most intriguing result concerns potential use of
noble gases to assess multiple gas sources and processes of gas hydrate
formation. We discuss this latter issue first. Then, with this background, we
return to our original questions.
Multiple Gas
Sources
The most basic result of
this study is that our gas samples contain at least three distinct sources of
noble gases. This conclusion is best shown by plotting one F(i) value
against another for all eight gas samples (Fig.
3, Fig. 4). Mixing of
two gases with different noble gas compositions will result in gas samples that
plot as a line on an F(i) vs. F(i) diagram. Mixing of two
end-member gases cannot explain the array of F(i) values for our
samples (Fig. 3, Fig.
4). A plot of F(4He) vs. R/Ra for all eight
samples also indicates multiple gas sources (Fig.
5).
The simplest explanation
for the observed noble gas compositions is that there are three gases, and
samples are mixtures of two of these gases (Fig.
3, Fig. 4, Fig.
5). Gas A is similar to air but depleted in 3He; it has a low
R/Ra (<0.2), low F(4He) (<25), and fractionation
factors for 4Ne, 86Kr, and 132Xe that are
approximately 1. Relative to air, Gas B is depleted in Ne but enriched in Kr and
Xe; it has an R/Ra and F(4He) of approximately 1, a
moderately low F(22Ne) (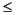 0.7),
a moderately high F(86Kr) (>1.9), and a very high F(132Xe)
(>17). Relative to air, Gas C is very depleted in Ne but enriched in He, Kr,
and Xe; it has an R/Ra <1.0, a relatively high F(4He)
(>300), a low F(22Ne) (~0.4), a high F(86Kr) (>2.5),
and a moderately high F(132Xe) (>7).
0.7),
a moderately high F(86Kr) (>1.9), and a very high F(132Xe)
(>17). Relative to air, Gas C is very depleted in Ne but enriched in He, Kr,
and Xe; it has an R/Ra <1.0, a relatively high F(4He)
(>300), a low F(22Ne) (~0.4), a high F(86Kr) (>2.5),
and a moderately high F(132Xe) (>7).
Hypothesized Gas A
comprises a significant component of six and probably all of the samples (Fig.
3, Fig. 4, Fig.
5) and likely reflects air contamination. Three pieces of information
lead to this conclusion. First, samples that were most susceptible to slow gas
exchange with air appear to have the most air. The volume of gas released from a
hydrate specimen (Table 2) is
proportional to the initial pressure inside the stainless steel bottle (except
for the B split of Section 164-997A-42X-3, which had a low pressure). Samples
with low gas volumes (low pressures) or long storage times (seven months) have
compositions most similar to Gas A. Second, combining the 36Ar
concentrations measured in the gas phase (Table
3) with the gas/water ratios for the samples (Table
2) results in 36Ar concentrations per cubic centimeter water
that are ~8-73 times more than that expected for air-saturated seawater. Third,
concentrations of N2 and O2 (i.e., air) in our gas samples
as determined on ship and before shipping were below detection limits,
suggesting that air was probably introduced to the stainless steel bottles
during storage and shipping. Assuming that Gas A is air and an end-member
component of our samples, the depletion of 3He in Gas A relative to
air (Fig. 5) is consistent with
mixing the 4He-enriched sample gas with air, as depicted by the
dashed mixing line in Figure 5.
Hypothesized Gas B
comprises a significant component of Sample 997A-42X-3(1B) and, perhaps, Samples
996B-1H-1(1), 996B-1H-1(2), 997A-42X-3(1A), and 994C-31X-7(1) (Fig.
3, Fig. 4, Fig.
5). If we make two assumptions, relative ratios of Ar, Kr, and Xe in Gas
B are similar to those expected in the clathrate lattice after formation of
hydrate from water, CH4, and air near 0ºC. The two assumptions are:
(1) noble gas fractionation is similar for all gas-water-hydrate systems (e.g.,
CH3Cl and CH4) where the solid product is a Structure I
clathrate (Table 1), and (2)
noble gas fractionation at three-phase free gas-dissolved gas hydrate
equilibrium conditions (Table 1)
is similar to combined fractionation between free gas and dissolved gas and
between dissolved gas and hydrate. The second assumption arises because natural
gas hydrate probably forms from water in the absence of free gas, but
experimental work concerning noble gas incorporation into hydrates was conducted
under conditions where hydrate was in equilibrium with free gas. We speculate
that noble gases of Gas B enter the clathrate lattice during formation of CH4
hydrate from CH4 and air-saturated seawater when CH4
quantities surpass the partial saturation curve for CH4 hydrate (Zatsepina
and Buffett, 1997).
Hypothesized Gas C
comprises a significant component of Sample 996E-7H-CC(1) and, to a lesser
degree, Samples 996C-1H-1(1) and 996C-1H-1(2) (Fig.
3, Fig. 4, Fig.
5). The noble gas composition of Gas C is not air, and it is unlike the
expected composition of gas in the hydrate lattice after hydrate formation from
water, CH4, and air.
We cannot constrain the
source of Gas B and Gas C without additional and less contaminated samples.
However, although we cannot conclude that there are only two sources (other than
air contamination) of noble gases in gas hydrate on the Blake Ridge, there must
be at least two, and each might be associated with a different flow regime. Gas
B is defined by Sample 997A-42X-3(1B) collected in deep sediment in an area of
limited fluid flow, whereas Gas C is defined by Sample 996E-7H-CC(1) collected
in shallow sediment in a region of active fluid venting. Unfortunately, the
extent the two flow regimes interact is not discernible from the present data
set. The noble gases suggest that there may be at least two different pathways
for the formation of CH4 hydrate on the Blake Ridge, a conclusion
realized by others (Paull, Matsumoto, Wallace, et al., 1996; Egeberg and
Dickens, 1999) and compatible with the observation of at least two distinct
fluid flow regimes.
Gas Hydrate
Detection in Gas Fields
Verkhovsky et al. (1988)
presented noble gas analyses for two gas samples collected from wells of the
Messoyakha gas field in a permafrost region of Siberia. These analyses are
intriguing because the samples have F(132Xe) < F(86Kr)
< 1 (Fig. 6), a result
unlike samples recovered from other gas fields where F(132Xe) > F(86Kr)
> 1 (e.g., Verkhovsky et al., 1988; Hiyagon and Kennedy, 1992).
The Messoyakha field is
known to contain abundant natural gas hydrate predominantly composed of CH4
(Makogon, 1981). This hydrate component distinguishes the Messoyakha field from
other exploited gas fields around the world. Verkhovsky et al. (1988) therefore
suggested that depletion of Kr and Xe in their samples is caused by
fractionation during hydrate formation such that residual gas collected at wells
is depleted in heavier noble gases. These authors then suggested that noble
gases could be used to detect the presence (or absence) of gas hydrate in a gas
field.
Implicit in the proposals
of Verkhovsky et al. (1988) is that natural gas hydrate in a gas field would
have F(132Xe) > F(86Kr) > the water from which the
hydrate forms, and therefore greater than ~2-4 (e.g., Table
3). Although we do not know the detailed pathways of noble gas
incorporation into hydrate on the Blake Ridge, samples that are the least air
contaminated have F(132Xe) > F(86Kr) > air-saturated
seawater. Our analyses provide support (albeit limited) for the ideas of
Verkhovsky et al. (1988). Obviously, the next step for this particular avenue of
research would be to determine the noble gas composition of residual gas and gas
hydrate in the same gas field. If low F(Xe) values presented by Verkhovsky et
al. (1988) are from a residual pore fluid in a hydrated zone, then the
hydrate-pore fluid system must be closed, as suggested by Claypool and Kaplan
(1974), or at least partly closed, as argued by Egeberg and Dickens (1999).
Global Xenon
Inventory
The current
estimate for the mass of natural gas hydrate in sediment along continental
margins is 7.5 to 15 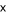 1018 g of
carbon as CH4 (Kvenvolden, 1993; Gornitz and Fung, 1994). Our gas
sample that is most enriched in Xe (Sample 997A-42X-3[1B]) consists of 98.4% CH4
with an F(132Xe) value of 17.8 (Table
2, Table 3), or a total Xe
concentration of 0.22 ppmv (using noble gas concentrations and isotope
abundances from Ozima and Podosek, 1983). Thus, the mass ratio of Xe to CH4
in this particular sample is ~2
1018 g of
carbon as CH4 (Kvenvolden, 1993; Gornitz and Fung, 1994). Our gas
sample that is most enriched in Xe (Sample 997A-42X-3[1B]) consists of 98.4% CH4
with an F(132Xe) value of 17.8 (Table
2, Table 3), or a total Xe
concentration of 0.22 ppmv (using noble gas concentrations and isotope
abundances from Ozima and Podosek, 1983). Thus, the mass ratio of Xe to CH4
in this particular sample is ~2 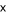 10-7.
Even if this Xe-enriched gas sample was representative of most natural CH4
hydrate along continental margins, the total inventory of Xe in oceanic CH4
hydrate would only be 1 to 3
10-7.
Even if this Xe-enriched gas sample was representative of most natural CH4
hydrate along continental margins, the total inventory of Xe in oceanic CH4
hydrate would only be 1 to 3 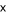 1013
g. This quantity of Xe is ~100 times less than the amount of Xe in the
atmosphere (2.02
1013
g. This quantity of Xe is ~100 times less than the amount of Xe in the
atmosphere (2.02 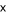 1015 g; Ozima and
Podosek, 1983). Oceanic gas hydrate is preferentially enriched in Xe but by an
amount that is insufficient to account for any of our Earth's "missing
xenon."
1015 g; Ozima and
Podosek, 1983). Oceanic gas hydrate is preferentially enriched in Xe but by an
amount that is insufficient to account for any of our Earth's "missing
xenon."

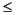 0.7),
a moderately high F(86Kr) (>1.9), and a very high F(132Xe)
(>17). Relative to air, Gas C is very depleted in Ne but enriched in He, Kr,
and Xe; it has an R/Ra <1.0, a relatively high F(4He)
(>300), a low F(22Ne) (~0.4), a high F(86Kr) (>2.5),
and a moderately high F(132Xe) (>7).
0.7),
a moderately high F(86Kr) (>1.9), and a very high F(132Xe)
(>17). Relative to air, Gas C is very depleted in Ne but enriched in He, Kr,
and Xe; it has an R/Ra <1.0, a relatively high F(4He)
(>300), a low F(22Ne) (~0.4), a high F(86Kr) (>2.5),
and a moderately high F(132Xe) (>7).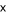 1018 g of
carbon as CH4 (Kvenvolden, 1993; Gornitz and Fung, 1994). Our gas
sample that is most enriched in Xe (Sample 997A-42X-3[1B]) consists of 98.4% CH4
with an F(132Xe) value of 17.8 (
1018 g of
carbon as CH4 (Kvenvolden, 1993; Gornitz and Fung, 1994). Our gas
sample that is most enriched in Xe (Sample 997A-42X-3[1B]) consists of 98.4% CH4
with an F(132Xe) value of 17.8 (