The ![]() 18O
values in the upper portion of the sediments in conjunction with the uniform
concentration of Sr2+ and Cl- (Table
6; Fig. 6) in the pore fluids
support our ideas of active fluid movement in this portion of the profile
(Shipboard Scientific Party, 1997). It is also possible that a period with a
very high rate of sedimentation could have produced a pattern in the pore-water
geochemistry similar to that observed. In order for this mechanism to be
responsible, the entire thickness of the flushed zone must have been deposited
over a geologically short period of time. Although this mechanism is unlikely
based on the present rates of sedimentation (Shipboard Scientific Party, 1997),
it is possible that rates of deposition are outside the range of the
chronostratigraphic markers used in this study. Some support for this idea might
be provided by the presence of a thinner flushed zone in Sites 1003 and 1007,
which shows depositional rates of ~5 cm/k.y. over the upper 50 mbsf compared to
10-15 cm/k.y. at Sites 1003 through 1005.
18O
values in the upper portion of the sediments in conjunction with the uniform
concentration of Sr2+ and Cl- (Table
6; Fig. 6) in the pore fluids
support our ideas of active fluid movement in this portion of the profile
(Shipboard Scientific Party, 1997). It is also possible that a period with a
very high rate of sedimentation could have produced a pattern in the pore-water
geochemistry similar to that observed. In order for this mechanism to be
responsible, the entire thickness of the flushed zone must have been deposited
over a geologically short period of time. Although this mechanism is unlikely
based on the present rates of sedimentation (Shipboard Scientific Party, 1997),
it is possible that rates of deposition are outside the range of the
chronostratigraphic markers used in this study. Some support for this idea might
be provided by the presence of a thinner flushed zone in Sites 1003 and 1007,
which shows depositional rates of ~5 cm/k.y. over the upper 50 mbsf compared to
10-15 cm/k.y. at Sites 1003 through 1005.
Within the reproducibility
of the replicate ![]() 18O
analyses and the standard deviation of our standards, there appears to be some
variation in the upper portions of the flushed zone that is not evident in the
Cl- data. It is possible that this variability is real;
alternatively, it is possible that it results from nonuniformity in the manner
in which the samples were sealed. The increasing
18O
analyses and the standard deviation of our standards, there appears to be some
variation in the upper portions of the flushed zone that is not evident in the
Cl- data. It is possible that this variability is real;
alternatively, it is possible that it results from nonuniformity in the manner
in which the samples were sealed. The increasing ![]() 18O
of the pore waters in the flushed zone closer to the margin of the carbonate
platform probably indicates that the bottom waters at these sites originated
from the surface of the Great Bahama Bank—perhaps during the winter, when
cold, dense saline water is known to cascade over the margin.
18O
of the pore waters in the flushed zone closer to the margin of the carbonate
platform probably indicates that the bottom waters at these sites originated
from the surface of the Great Bahama Bank—perhaps during the winter, when
cold, dense saline water is known to cascade over the margin.
The increase in the
concentration of chloride with increasing depth noted at all the sites during
Leg 166 was postulated to be a result of the diffusion of Cl- from an
underlying evaporitic source (Shipboard Scientific Party, 1997). Such a source
was drilled at Site 627, north of Little Bahama Bank (Austin, Schlager, et al.,
1986), and pore waters showed a similar increase in Cl- approaching
this source (Swart and Guzikowski, 1988). However, analysis of the Na+/Cl-
ratios of the interstitial pore-water data from Leg 166 reveals no evidence of a
NaCl source. Perhaps a more likely origin is simply evaporated seawater that has
become entrapped in rocks during sea-level low stands. However, the evaporated
seawater origin is not supported by absence of a correlation between ![]() 18O
and
18O
and ![]() D.
Therefore, the origin of the increase in salinity is still an enigma: whereas
the absence of a correlation between
D.
Therefore, the origin of the increase in salinity is still an enigma: whereas
the absence of a correlation between ![]() 18O
and
18O
and ![]() D
(Fig. 11) suggests the
dissolution of NaCl, the absence of a change in the Na/Cl ratio with increasing
depth tends to favor the presence of a deep-seated saline fluid.
D
(Fig. 11) suggests the
dissolution of NaCl, the absence of a change in the Na/Cl ratio with increasing
depth tends to favor the presence of a deep-seated saline fluid.
Typically, pore waters in
deep-sea sediments show a decrease in their ![]() 18O
values as a result of the interaction between pore water and igneous basement
rocks (Lawrence, 1989). In the Bahamas, because the influence of interactions
with basement rocks is likely to be minimal at the depths drilled in this study,
the observed increase in
18O
values as a result of the interaction between pore water and igneous basement
rocks (Lawrence, 1989). In the Bahamas, because the influence of interactions
with basement rocks is likely to be minimal at the depths drilled in this study,
the observed increase in ![]() 18O
at most sites is best explained as a result of the recrystallization of biogenic
carbonate components in the presence of a geothermal gradient. The magnitude of
the expected changes resulting from recrystallization of carbonates will depend
upon the amount of recrystallization, the geothermal gradient, and the
18O
at most sites is best explained as a result of the recrystallization of biogenic
carbonate components in the presence of a geothermal gradient. The magnitude of
the expected changes resulting from recrystallization of carbonates will depend
upon the amount of recrystallization, the geothermal gradient, and the ![]() 18O
of the initial sediment and pore water. Using the equations presented by
previous researchers (Lawrence, 1973, 1989; Lawrence et al., 1976; Killingley,
1983; Stout, 1985), the isotopic composition of the pore water after a specified
amount of recrystallization can be calculated from the following equation:
18O
of the initial sediment and pore water. Using the equations presented by
previous researchers (Lawrence, 1973, 1989; Lawrence et al., 1976; Killingley,
1983; Stout, 1985), the isotopic composition of the pore water after a specified
amount of recrystallization can be calculated from the following equation:
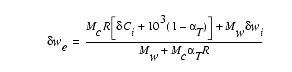 (1)
(1)
In this equation:
As an example, Figure
12 shows the evolution of the interstitial water (![]() we)
in the presence of various different conditions that are likely to occur during
burial. Case A represents a geothermal gradient of 50°C/km, an initial sediment
we)
in the presence of various different conditions that are likely to occur during
burial. Case A represents a geothermal gradient of 50°C/km, an initial sediment
![]() 18O
value of +0.0
18O
value of +0.0![]() Peedee
belemnite (PDB), an initial water
Peedee
belemnite (PDB), an initial water ![]() 18O
value of 0.5
18O
value of 0.5![]() SMOW, and
a recrystallization rate of 1% per 5 m. Case B represents the same conditions
with the exception of a geothermal gradient of 70°C/km. Case C represents the
same conditions as Case A except that the initial oxygen isotopic composition of
the water is +0.5
SMOW, and
a recrystallization rate of 1% per 5 m. Case B represents the same conditions
with the exception of a geothermal gradient of 70°C/km. Case C represents the
same conditions as Case A except that the initial oxygen isotopic composition of
the water is +0.5![]() . In
all cases shown in Figure 12,
the
. In
all cases shown in Figure 12,
the ![]() 18O
increases to values consistent with those observed at the Leg 166 sites.
18O
increases to values consistent with those observed at the Leg 166 sites.
The only exception to the
trend of increasing pore-water ![]() 18O
with increasing depth was seen at Site 1006 (Fig.
10). The absence of an increase in
18O
with increasing depth was seen at Site 1006 (Fig.
10). The absence of an increase in ![]() 18O
at this site is probably a consequence of reduced amounts of carbonate
diagenesis compared to the other more proximal locations (Eberli, Swart, Malone,
et al., 1997; Shipboard Scientific Party, 1997). The relative absence of
carbonate diagenesis is evident from the excellent preservation of the
foraminifers and nannofossils, which made this site a particularly good one for
biostratigraphic purposes (Shipboard Scientific Party, 1997).
18O
at this site is probably a consequence of reduced amounts of carbonate
diagenesis compared to the other more proximal locations (Eberli, Swart, Malone,
et al., 1997; Shipboard Scientific Party, 1997). The relative absence of
carbonate diagenesis is evident from the excellent preservation of the
foraminifers and nannofossils, which made this site a particularly good one for
biostratigraphic purposes (Shipboard Scientific Party, 1997).
Some support for water
being drawn into the platform was provided by unusual chloride profiles,
particularly at Sites 1004 and 1005 (Fig.
3). These profiles suggested that less-saline bottom water was being
drawn into the platform, perhaps by a mechanism such as Kohout convection (Kohout,
1966; Simms, 1984). An alternative scenario is that more-saline water,
originating from the platform top, is sinking downward through the upper
sediments. Although the anomaly in the chloride was also present as slightly
depressed ![]() 18O
values, the data are consistent with either explanation for the origin of the Cl-anomaly.
18O
values, the data are consistent with either explanation for the origin of the Cl-anomaly.