IGNEOUS MINERAL CHEMISTRY
The analyzed samples and the resultant average mineral analyses are summarized in Table T1. More compositional details are given in Table T2 (plagioclase), Table T3 (clinopyroxene and orthopyroxene), Table T4 (olivine), Table T5 (titanomagnetite and ilmenite), Table T6 (apatite), and Table T7
(biotite).
Plagioclase shows extensive solid solutions from An70Ab30 to An30Ab69 concurrently with a systematic increase in potassium from Or0.4 to Or1.0 (Fig. F11). The iron content of plagioclase is relatively low (FeO = 0.21 wt%) and only weakly increases with Ab content. The systematic variations in Ab and Or point toward a cogenetic suite from olivine gabbros to Fe-Ti oxide gabbros, gabbronorites, and apatite gabbros.
Despite the limited preservation and restricted appearance of olivine, its composition parallels the evolution of plagioclase. Olivine shows strong iron enrichment and varies in composition from Fo88 to Fo35 in the general sequence from olivine gabbros to Fe-Ti oxide gabbros and apatite gabbros (Fig. F12). There are two intervals of olivine crystallization with a gap between approximately Fo46 and Fo40. This interval without olivine crystallization appears to correspond to the appearance of orthopyroxene. The most fayalitic olivine is found in the apatite gabbros (Fo36-Fo35). Manganese shows a systematic increase with increasing fayalite content of the olivine (Fig. F12), but other analyzed minor elements (Ni and Cr) show no systematic variation.
Extensive variation is revealed by the clinopyroxene compositions in both minor and major elements. The quadrilateral components (94%-91%) (Fig. F13) define the clinopyroxenes as dominantly augitic to slightly ferroaugitic (Poldervaart and Hess, 1951). Pyroxene quadrilateral geothermometry suggests equilibration temperatures from 1100°C and down to 700°C (Fig. F13) (Lindsley, 1983).
The nonquadrilateral cations include Na, Al, Mn, Ti, and Cr (Fig. F14). Chromium shows a strong decrease for the olivine gabbros with decreasing Mg/(Mg + Fe2+) ratio but remains low in the Fe-Ti oxide-bearing gabbros. Manganese shows systematic increases and Al shows a systematic decrease with decreasing Mg/(Mg + Fe2+) ratios throughout the gabbro suite. Despite large variation, Ti shows an increase in the olivine gabbros but indicates a moderate decrease in the Fe-Ti oxide-bearing gabbros. This break in the Ti variation is also revealed by the Ti/Al ratios, for which the olivine gabbros suggest an increase and the Fe-Ti oxide gabbros suggest relatively constant ratios (Fig. F15).
The major elements show a strong negative correlation between Mg and Fe2+ and only a small decrease in Ca with increasing Fe2+ (Fig. F16). The Mg and Fe2+ variation deviates from the trend expected from a dominating Fe2+ 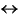 Mg cation exchange. The deviation increases with increasing Fe2+, in part due to (Mg, Fe2+)
Mg cation exchange. The deviation increases with increasing Fe2+, in part due to (Mg, Fe2+) 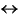 Ca exchange as well as coupled exchange mechanisms such as TiAl2
Ca exchange as well as coupled exchange mechanisms such as TiAl2 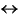 (Mg, Fe2+)Si2. The exchange mechanisms can be further evaluated by assigning cations to likely site positions in the pyroxene structure. The pyroxene compositions have been recalculated with all Si assigned to tetrahedral position and Fe3+, Ti, and Cr to the M1 position in the structure. The Al is distributed between tetrahedral and M1 positions, assuming that the total amounts of tetrahedral cations is 2. Ca and Na occur only in M2 positions, and Fe2+, Mn, and Mg may occur in either M1 or M2 octahedral positions (Lindsley and Andersen, 1983). The distribution of iron between Fe2+ and Fe3+ has been estimated based on charge balancing the cations (Papike et al., 1974). The results suggest Fe3+/Fe2+ ratios of 0.4-0.1, with a systematic fall in the average values for each group of gabbros from olivine gabbro to Fe-Ti oxide gabbro and apatite gabbro. Both tetrahedral AlIV and octahedral AlVI slightly decrease in the same sequence. The Ti vs. AlIV variation suggests wide variations in the Ti/Al ratios from between 1:2 and 1:8 (Fig. F15). The Na content is relatively constant without any systematic dependence on Fe or Mg contents. Based on simplified partitionings, the nonquadrilateral cations can be broken down as aegerine (2.6%-2.9%; NaFe3+Si2O6), jadeite (0.5%-0.7%; NaAlSi2O6), fassaite (1.3%-1.7%; CaFe3+AlSiO6), Ti-tschermakite (1.3%-1.9%; CaTiAl2O6), and Ca-tschermakite (0.8%-2.8%, CaAlAlSiO6) (Lindsley, 1983). These components show little systematic variation with the quadrilateral components, with the exception of Ti-tschermakite, which shows a maximum for an intermediate ferrosilite composition.
(Mg, Fe2+)Si2. The exchange mechanisms can be further evaluated by assigning cations to likely site positions in the pyroxene structure. The pyroxene compositions have been recalculated with all Si assigned to tetrahedral position and Fe3+, Ti, and Cr to the M1 position in the structure. The Al is distributed between tetrahedral and M1 positions, assuming that the total amounts of tetrahedral cations is 2. Ca and Na occur only in M2 positions, and Fe2+, Mn, and Mg may occur in either M1 or M2 octahedral positions (Lindsley and Andersen, 1983). The distribution of iron between Fe2+ and Fe3+ has been estimated based on charge balancing the cations (Papike et al., 1974). The results suggest Fe3+/Fe2+ ratios of 0.4-0.1, with a systematic fall in the average values for each group of gabbros from olivine gabbro to Fe-Ti oxide gabbro and apatite gabbro. Both tetrahedral AlIV and octahedral AlVI slightly decrease in the same sequence. The Ti vs. AlIV variation suggests wide variations in the Ti/Al ratios from between 1:2 and 1:8 (Fig. F15). The Na content is relatively constant without any systematic dependence on Fe or Mg contents. Based on simplified partitionings, the nonquadrilateral cations can be broken down as aegerine (2.6%-2.9%; NaFe3+Si2O6), jadeite (0.5%-0.7%; NaAlSi2O6), fassaite (1.3%-1.7%; CaFe3+AlSiO6), Ti-tschermakite (1.3%-1.9%; CaTiAl2O6), and Ca-tschermakite (0.8%-2.8%, CaAlAlSiO6) (Lindsley, 1983). These components show little systematic variation with the quadrilateral components, with the exception of Ti-tschermakite, which shows a maximum for an intermediate ferrosilite composition.
The low-Ca pyroxene is an orthopyroxene with generally <4% wollastonite and Mg/(Mg + Fetotal) ratios narrowly between 0.49 and 0.51 (Fig. F13). The apparent closure of the gap between Ca-rich and Ca-poor pyroxenes, seen on Figure F13 and also reflected on Figure F16 for analyses with high Mg and low Ca, in part reflects the inability of the broad analytical microbeam technique always to consistently average unexsolved pyroxene composition.
There is no detectable difference in the compositions of the orthopyroxenes in the apatite-bearing and the apatite-free gabbros. The nonquadrilateral elements are mostly Ti and Al (Fig. F14) and amount to <2% of the total. The exchange coefficient for Mg and Fe between the two pyroxenes ([Mg/Fe]cpx/[Mg/Fe]opx) range between 0.500 and 0.763 (N = 5), mainly due to variation in the coexisting augites. As a result, the slope of the tie-lines between coexisting orthopyroxene and augite (Fig. F13) appears to correspond better to experimentally determined augite-pigeonite than to augite-orthopyroxene pairs (Lindsley, 1983). Therefore, it is possible that the orthopyroxenes reflect subsolidus inversion or granule exsolution from high-temperature pigeonite.
The Fe-Ti oxide minerals of the oxide gabbros are present as granular intergrowth between ilmenite and titanomagnetite. The ilmenite is generally free of hematite lamellae, whereas titanomagnetite often contains lamellar to granule exsolution of titanomagnetite. The analytical technique attempted to incorporate the exsolved component by using a broad beam and by analyzing the relatively unexolved parts of the grains. Figure F17 illustrates the extent of solid solution in the two Fe-Ti oxide minerals as well as the range of tie-lines between coexisting titanomagnetite and ilmenite. The minor elements in the two Fe-Ti oxide series show little correlation with rock types (Fe-Ti oxide or apatite gabbros). The only exception is Mn, which is negatively correlated with Mg content of ilmenite (Fig. F18). The ilmenite in the apatite gabbros has the highest Mn content. This is consistent with the increase of Mn seen in olivine and augite with increasing Fe2+ or decreasing Mg/(Mg + Fe2+) ratio.
The compositions of the coexisting ilmenite and titanomagnetite can be used to calculate the equilibration temperature and oxygen fugacity (fO2) (Buddington and Lindsley, 1964; Andersen and Lindsley, 1988). The result of these calculations for average compositions of individual samples is shown in Figure F19. The equilibration temperatures range from 850° to 675°C, and the apatite gabbros have the lowest equilibrium temperatures. These results generally correspond to the equilibrium temperatures determined for augite (1100°-700°C) and suggest that the Fe-Ti oxide pairs continued to reequilibrate past closure of the silicate assemblage. The calculated oxygen fugacity clusters around the fayalite-magnetite-quartz (FMQ) oxygen buffer at relatively high temperatures, but deviates for lower temperatures vary by up to 0.5 log units below the FMQ.
Apatite contains various amounts of anionic fluorine, chlorine, and hydroxyl. The fluorine content is >2.6 wt%, and the chlorine content is <0.40 wt%. The OH content can be estimated from the deficiency in the total cations (F + Cl + OH = 2) and is <30% of the total anions. Cations in minor concentrations (Fe, Mn, Mg, and Na) are generally well below 0.2 wt%. The solid solutions of the apatites are illustrated in Figure F20 in terms of the F + Cl + OH component as dominantly flourapatites to hydroxyapatites with minor amounts of chlorapatite.
Mica was detected in one of the most evolved apatite gabbros (Fo32) as a few grains of likely primary igneous origin. The average composition is illustrated in Table T7 and defines the mica as a typical biotite with a Mg/Fe ratio of 1:2.

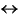 Mg cation exchange. The deviation increases with increasing Fe2+, in part due to (Mg, Fe2+)
Mg cation exchange. The deviation increases with increasing Fe2+, in part due to (Mg, Fe2+) 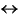 Ca exchange as well as coupled exchange mechanisms such as TiAl2
Ca exchange as well as coupled exchange mechanisms such as TiAl2 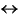 (Mg, Fe2+)Si2. The exchange mechanisms can be further evaluated by assigning cations to likely site positions in the pyroxene structure. The pyroxene compositions have been recalculated with all Si assigned to tetrahedral position and Fe3+, Ti, and Cr to the M1 position in the structure. The Al is distributed between tetrahedral and M1 positions, assuming that the total amounts of tetrahedral cations is 2. Ca and Na occur only in M2 positions, and Fe2+, Mn, and Mg may occur in either M1 or M2 octahedral positions (Lindsley and Andersen, 1983). The distribution of iron between Fe2+ and Fe3+ has been estimated based on charge balancing the cations (Papike et al., 1974). The results suggest Fe3+/Fe2+ ratios of 0.4-0.1, with a systematic fall in the average values for each group of gabbros from olivine gabbro to Fe-Ti oxide gabbro and apatite gabbro. Both tetrahedral AlIV and octahedral AlVI slightly decrease in the same sequence. The Ti vs. AlIV variation suggests wide variations in the Ti/Al ratios from between 1:2 and 1:8 (Fig. F15). The Na content is relatively constant without any systematic dependence on Fe or Mg contents. Based on simplified partitionings, the nonquadrilateral cations can be broken down as aegerine (2.6%-2.9%; NaFe3+Si2O6), jadeite (0.5%-0.7%; NaAlSi2O6), fassaite (1.3%-1.7%; CaFe3+AlSiO6), Ti-tschermakite (1.3%-1.9%; CaTiAl2O6), and Ca-tschermakite (0.8%-2.8%, CaAlAlSiO6) (Lindsley, 1983). These components show little systematic variation with the quadrilateral components, with the exception of Ti-tschermakite, which shows a maximum for an intermediate ferrosilite composition.
(Mg, Fe2+)Si2. The exchange mechanisms can be further evaluated by assigning cations to likely site positions in the pyroxene structure. The pyroxene compositions have been recalculated with all Si assigned to tetrahedral position and Fe3+, Ti, and Cr to the M1 position in the structure. The Al is distributed between tetrahedral and M1 positions, assuming that the total amounts of tetrahedral cations is 2. Ca and Na occur only in M2 positions, and Fe2+, Mn, and Mg may occur in either M1 or M2 octahedral positions (Lindsley and Andersen, 1983). The distribution of iron between Fe2+ and Fe3+ has been estimated based on charge balancing the cations (Papike et al., 1974). The results suggest Fe3+/Fe2+ ratios of 0.4-0.1, with a systematic fall in the average values for each group of gabbros from olivine gabbro to Fe-Ti oxide gabbro and apatite gabbro. Both tetrahedral AlIV and octahedral AlVI slightly decrease in the same sequence. The Ti vs. AlIV variation suggests wide variations in the Ti/Al ratios from between 1:2 and 1:8 (Fig. F15). The Na content is relatively constant without any systematic dependence on Fe or Mg contents. Based on simplified partitionings, the nonquadrilateral cations can be broken down as aegerine (2.6%-2.9%; NaFe3+Si2O6), jadeite (0.5%-0.7%; NaAlSi2O6), fassaite (1.3%-1.7%; CaFe3+AlSiO6), Ti-tschermakite (1.3%-1.9%; CaTiAl2O6), and Ca-tschermakite (0.8%-2.8%, CaAlAlSiO6) (Lindsley, 1983). These components show little systematic variation with the quadrilateral components, with the exception of Ti-tschermakite, which shows a maximum for an intermediate ferrosilite composition.