-
Ca2+ + CO32-
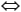 CaCO3(s) and
CaCO3(s) and
- Ca2+ + Mg2+ + 2CO32-
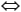 CaMg(CO3)2(s).
CaMg(CO3)2(s).
- Ca2+ + Mg2+ + 2CO32-
Interstitial water samples of Hole 1127B were taken at a rate of two per core for the first five cores and one per core thereafter. Samples were analyzed according to the procedures outlined in "Inorganic Geochemistry" in the "Explanatory Notes" chapter. The data are presented in Table T6 and Figures F14, F15, F16, F17, and F18.
Salinity shows a linear increase from 34, reaching 88 by 239.3 mbsf. From 239.3 to 335.5 mbsf, salinity values are variable; below 335.5 mbsf, the values become more stable and reach a maximum of 96 (Fig. F14). Despite procedures taken to eliminate H2S from the samples (see "Inorganic Geochemistry" in the ""Explanatory Notes" chapter), determination of chlorinity at this site was hampered by the high concentration of H2S, which interfered with the endpoint determination of the titration. However, as Cl- was also determined using an ion chromatograph, we used the Cl- data measured by ion chromatography for interpretative purposes.
Concentrations of Ca2+ and Mg2+ decrease with depth from 11.3 and 54.6 mM, respectively, to 3.5 and 26.4 mM at a depth of 114.7 mbsf (Fig. F15). Below this depth, both elements show a steady increase in concentration, reaching values of 41.2 and 105.0 mM at 484.6 mbsf. The values of K+ show a steady increase, reaching a maximum at 427.4 mbsf. The concentrations of Li+ increase steadily from 36 to 590 然 at 484.6 mbsf, although the increase appears to have three distinct slopes (0-40, 40-200, and 200-482 mbsf). Silica increases more than threefold from 191 to 672 然 within the first 12 m of Hole 1127B and thereafter increases slowly, reaching a maximum of 970 然 at 104.54 mbsf. Below this depth, the values remain almost constant at a level of 900 然. The concentration of Sr2+ increases slowly from 73 to 200 然 at 151.8 mbsf. Below this depth, Sr2+ exhibits a marked increase to as much as 700 然 at 258.5 mbsf.
The concentration of NH4+ increases in the upper part of Hole 1127B, from 0.5 mM to 18.5 mM at 67.3 mbsf, and then remains constant (with the exception of a distinct peak between 150 and 200 mbsf) until 280 mbsf (Fig. F16). Below this depth, the NH4+ concentration steadily decreases to 5 mM at 481.7 mbsf. The phosphate data are very erratic and could have become contaminated during sample handling. However, in general, there is a constant increase in phosphate concentration to a maximum of 134.0 然 at 104.5 mbsf, followed by a decrease in concentration to values averaging 22 然 between 300 and 460 mbsf. The concentration of SO42- decreases from 30.4 mM to the detection limit within the upper 20 mbsf. Variable amounts of SO42- evident between 21.4 and 168.9 mbsf are probably a result of core contamination during retrieval by surface seawater circulated during drilling. Below 177.5 mbsf, the concentration of SO42- increases again, reaching 47.1 mM at 484.6 mbsf. Alkalinity increases rapidly from 3.3 to 31.7 mM between 4.5 and 8.9 mbsf. Below this depth, alkalinity displays a linear increase to 100 mM at 100 mbsf. Below 160 mbsf, alkalinity slowly decreases to 22 mM at 484.6 mbsf. The pH measured using the punch-in electrode (ppH) shows a rapid initial decrease within the first 20 mbsf from 7.83 to 6.79. Below this depth, the values decrease linearly to a pH of 5.92 at 481.7 mbsf.
Pore-water chemistry at Site 1127 is dominated by an extended sulfate reduction zone that produces large amounts of H2S and HCO3-. As was the case with Site 1126 (see "Inorganic Geochemistry" in the "Site 1126" chapter), the interstitial waters at Site 1127 show a salinity increase of as much as ~100 in sediments of Pliocene age. However, as a result of the much thicker Pleistocene at Site 1127 (see "Biostratigraphy"), the supplying brine occurs at greater depths; thus, the salinity values increase more slowly.
The presence of the brine affected the interstitial water chemistry of Site 1127 in several ways. The most readily perceivable effect was an intense smell of H2S. Concentrations reached 138,026 ppm in gas voids sampled from Core 182-1127B-32X (see "Organic Geochemistry"). H2S originates as a by-product of the decomposition of organic matter under conditions in which sulfate is used as a terminal electron acceptor. Production of H2S ceases with increasing depth as a result of the exhaustion of either SO42- or organic material. Below the sulfate reduction zone, CO2 is used as an oxidant and methanogenesis is the most common method of breakdown of organic material (see "Organic Geochemistry"). At Site 1127, the extent of the sulfate reduction zone is enhanced by the presence of high-salinity pore fluids located in deeper strata that possess SO42- concentrations higher than those of normal seawater (Fig. F17). As a result of the high SO42- concentration in the underlying pore waters, the concentration gradient is larger than for normal seawater. Because of the relatively low iron content of the sediment, most of the H2S does not react to form iron sulfides and freely diffuses out of the sulfate reduction zone.
The process of sulfate reduction produces two moles of alkalinity for every mole of sulfate reduced, and, simplistically, a 2:1 relationship could be expected between alkalinity and sulfate. Although this is approximately the relationship observed (Fig. F18), it does not take into consideration the ~40 mM of Mg2+ and 8 mM of Ca2+ lost from the pore fluids, which would consume 96 mM of alkalinity if precipitated as either LMC or dolomite according to the following equations:
A possible explanation for the alkalinity anomaly is that part of the consumption of Mg2+ arises from the alteration of clay minerals, although this process usually increases the concentration of Ca2+, which was not observed at Site 1127. However, alkalinity can also be produced through the dissociation of H2S, and this contribution makes the alkalinity deficit even greater than observed.
Carbonate recrystallization is evident not only from pore-water parameters, such as reduced Ca2+ and Mg2+ and enhanced Sr2+, but also from cementation in the cores (see "Lithostratigraphy"). This is also reflected in the X-ray diffraction (XRD) results. X-ray diffraction analysis shows the presence of a small amount of dolomite throughout most of the core, with high concentrations at depths of 184.2, 247.99, and 305.8 mbsf (Table T7, also in ASCII format). It should be noted that the first major increase in the concentration of Sr2+, as well as the maximum concentration of Sr2+, coincides with the first and second dolomite peaks at 184.2 and 248.0 mbsf. The coincidence of the increase in Sr2+ with changes in mineralogy may suggest that the diagenetic changes are taking place at the present time.
The mineralogy at Site 1127 is dominated by variations between aragonite, LMC, and high-Mg calcite (HMC) (Table T7; Fig. F19). Quartz and dolomite show minor variations. Although the origin of these variations is not known at the present time, it is likely that the increase in the input of HMC occurs during sea-level highstands, whereas lowstands are dominated by higher concentrations of LMC (Droxler et al., 1983). Concentrations of dolomite appear to increase coincident with decreases in sea level. Toward the bottom of the cored section, the concentrations of both HMC and aragonite decrease, probably as a result of recrystallization of these metastable minerals. Notably, the first interval in which LMC is the dominant mineral occurs at 180 mbsf, coincident with increased lithification in the core (see "Lithostratigraphy").