LABORATORY METHODS
Sample Preparation
To prepare samples for X-ray diffraction (XRD) analyses, specimens of mud and mudstone were gently crumbled and placed in a glass beaker with 3% H2O2 for at least 24 hr until digestion of organic matter stopped. We then added 250 mL of 4 g/L sodium hexametaphosphate to disperse the clays. Further disaggregation was accomplished in an ultrasonic bath. Heavily indurated samples were exposed to additional crushing and repeated ultrasonic dispersal. Samples were then washed via centrifugation (using six 60-mL tubes per sample at 8200 rpm for 25 min), resuspended in 360 mL of deionized distilled water, washed again, and transferred in suspension to a 125-mL plastic bottle. After further dispersion with an ultrasonic cell disruptor, we separated the <2-µm fraction using a centrifuge at 1000 rpm for 2 min, 24 s. Slides were prepared for XRD as oriented aggregates to enhance the clay mineral basal reflections, using the filter-peel method with a 0.45-µm membrane. Slides were solvated in an ethylene glycol atmosphere overnight at 60°C to expand the smectite. The time elapsed between removal from the ethylene glycol chamber and scanning was <80 min to minimize evaporation.
X-Ray Diffraction Analysis
A Scintag PAD V X-ray diffractometer was used to scan slides from 3° to 23°2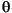 at the following parameters: radiation = CuK
at the following parameters: radiation = CuK ; scan rate = 1°/min; step size = 0.01; voltage = 40 kV; current = 30 mA; and slits = 0.2 mm. Data processing utilized MacDiff software (Petschick, 2001). Profiles were smoothed using the MacDiff standard weighted means 17-term filter. To correct for misalignments of the goniometer and slide holder, we shifted each diffractogram to realign the quartz (100) reflection at 4.26 Å.
; scan rate = 1°/min; step size = 0.01; voltage = 40 kV; current = 30 mA; and slits = 0.2 mm. Data processing utilized MacDiff software (Petschick, 2001). Profiles were smoothed using the MacDiff standard weighted means 17-term filter. To correct for misalignments of the goniometer and slide holder, we shifted each diffractogram to realign the quartz (100) reflection at 4.26 Å.
Relative Mineral Abundances
To estimate relative abundances of minerals in the clay-sized fraction by weight, we measured the integrated peak areas for basal reflections of smectite, illite, and chlorite, plus quartz. The targeted peaks are shown in Figure F5: smectite (001) at ~17 Å, illite (001) at ~10 Å, chlorite (002) at ~7 Å, and quartz (100) at 4.26 Å. Some difficulty with this method arises from peak overlap. The chlorite (001) peak occurs on the shoulder of the glycol-solvated smectite (001) peak at ~14 Å. More problematic is the complete overlap of chlorite (002) and kaolinite (001) peaks at ~7 Å. Although contents of kaolinite are probably small, we report the 7-Å values as chlorite (+ kaolinite). Interlayers of illite also affect the geometry of the smectite (001) peak, and the I/S (001)/(002) peak merges into the illite (001) peak as the percent illite in I/S increases. This interference adds counts from mixed-layer clay to peak-area values for both smectite and illite.
Another challenge in determining relative mineral abundance in natural sediments is selection of accurate weighting factors. One common method (Biscaye, 1965) multiplies the peak area of smectite (001) by 1x, illite (001) by 4x, and chlorite (002) by 2x, regardless of each mineral's abundance. The peak area generated by a given phase and reflection increases with that mineral's abundance, but each additional phase in a mixture also affects the intensity of all other reflections differently. Thus, to improve the accuracy of bulk powder analysis, Fisher and Underwood (1995) employed singular value decomposition (SVD) to solve for normalization factors using standard mineral mixtures with known weight percentages. Underwood et al. (this volume) solved for SVD weighting factors for the clay-sized fraction using a similar approach; they also completed a thorough analysis of error. We describe herein relative abundances (weight percent) of smectite, illite, chlorite, and quartz as calculated by SVD factors, but we also report the weighted peak areas for smectite, illite, and chlorite using Biscaye (1965) factors to make simplify comparisons with older data sets.
Chlorite and Kaolinite Peak Overlap
Previous workers showed that kaolinite is present in minor amounts at Site 808, ranging from 8% to 20% of the 7-Å peak area (Orr, 1992; see also Chamley et al., 1986). To extend the results of Orr (1992), we selected five representative samples from Sites 1173 and 1177 and boiled them for 2 hr in 1-N HCl. This treatment dissolves chlorite, so a peak remaining at 7 Å indicates the presence of kaolinite (Moore and Reynolds, 1997). Disappearance of the chlorite (003) reflection at 4.77 Å confirms the mineral's removal (Fig. F5). With MacDiff software, counts generated from treated and untreated specimens can be adjusted until the peak area for quartz (100) is the same for both. The ratio of untreated to HCl-treated (and adjusted) 7-Å peak areas then serves as a measure of the kaolinite contribution.
Percent Illite in Mixed-Layer Illite/Smectite Clays
Several methods exist for the XRD determination of percent illite in I/S mixed-layer clays. Srodon (1980) and Watanabe (1988) based their methods on differences in the positions of key peaks; however, they do not work well for samples with discrete illite. Another common method (Rettke, 1981) is based on the ratio of intensity of the "saddle" at the low diffraction angles to intensity of the 17-Å peak (Fig. F5). This method can be advantageous when samples contain small amounts of expandable clay, but it ceases to work when expandability drops below 40%. Reynolds and Hower (1970) documented the angular separation between I(001)/S(002) and I(002)/S(003) peaks (Fig. F5) using a combination of synthetic and natural diffractograms. Hathon (1992) compared this and other methods by imaging clays with transmission electron microscopy (TEM) and found that the percent smectite as estimated by XRD analyses usually falls within ±5% of visual estimates by TEM. Error in XRD data increases with the addition of discrete illite.
The advantage of using angular separation between two peaks is the method's insensitivity to instrument misalignments and variations in thickness of the ethylene glycol layer (Moore and Reynolds, 1997). If concentrations of I/S are low, however, all of the mixed-layer peaks are difficult to resolve above background counts, and in many cases the I(001)/S(002) peak is completely obscured. Results calculated from d-values of the I(002)/S(003) peak will match those based on separation between the two I/S peaks if each profile is corrected for misalignments. MacDiff software accomplishes this by shifting the quartz (100) peak to a d-value of 4.26 Å (although sometimes the correction command must be repeated). For many of the samples that we analyzed, the I(002)/S(003) peak is broad and irregular because of poor crystallinity and mixing among several types of I/S. The center of each peak was picked for its d-value by assuming a symmetrical shape.
As the smectite-illite transition proceeds, the percentage of illite in the mixed-layer structure increases and the organization of I/S interlayers changes from random to ordered (Reynolds and Hower, 1970; Altaner and Ylagan, 1997). I/S clays are usually disordered with 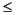 50% illite, and R = 1 ordering typically occurs at 55%-60% illite. The development of ordering may be recognized by a shift of the second-order I/S superstructure from ~5° to 6.5°2
50% illite, and R = 1 ordering typically occurs at 55%-60% illite. The development of ordering may be recognized by a shift of the second-order I/S superstructure from ~5° to 6.5°2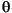 (Moore and Reynolds, 1997). During processing of XRD data, we also checked the position of this reflection.
(Moore and Reynolds, 1997). During processing of XRD data, we also checked the position of this reflection.
Smectite Type
We selected nine samples from Site 1173 and eight samples each from Sites 1174 and 1177 to identify the type of smectite. Because the (060) reflection is sensitive to the site occupancy and cation size in the octahedral sheet, its d-value allows dioctahedral varieties to be distinguished from trioctahedral varieties. The d(060) values for dioctahedral montmorillonite and beidellite are between 1.492 and 1.504 Å, whereas d-values for the trioctahedral forms (saponite and hectorite) and dioctahedral nontronite range from 1.520 to 1.530 Å (Brindley, 1980). Because (060) reflections are weak in most oriented clay aggregates, random powder mounts must be prepared. Portions of the <2-µm fraction were freeze-dried, manually disaggregated to remove clumps, and pressed into holders, taking care to minimize orientation of the particles. Samples were scanned from 48° to 64°2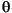 at scan rate = 0.5°/min, step size = 0.01, voltage = 40 kV, and current = 30 mA. This scanning range includes a (112) quartz peak at 1.817 Å, which permits corrections for small offsets of the goniometer (Fig. F5). The corrected position of the quartz (211) peak can also be verified at 1.542 Å ± 0.001 Å. The center of the (060) smectite peak was used to obtain its d-value, checking the profiles in both the smoothed and unsmoothed form (Fig. F5).
at scan rate = 0.5°/min, step size = 0.01, voltage = 40 kV, and current = 30 mA. This scanning range includes a (112) quartz peak at 1.817 Å, which permits corrections for small offsets of the goniometer (Fig. F5). The corrected position of the quartz (211) peak can also be verified at 1.542 Å ± 0.001 Å. The center of the (060) smectite peak was used to obtain its d-value, checking the profiles in both the smoothed and unsmoothed form (Fig. F5).

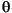 at the following parameters: radiation = CuK
at the following parameters: radiation = CuK ; scan rate = 1°/min; step size = 0.01; voltage = 40 kV; current = 30 mA; and slits = 0.2 mm. Data processing utilized MacDiff software (Petschick, 2001). Profiles were smoothed using the MacDiff standard weighted means 17-term filter. To correct for misalignments of the goniometer and slide holder, we shifted each diffractogram to realign the quartz (100) reflection at 4.26 Å.
; scan rate = 1°/min; step size = 0.01; voltage = 40 kV; current = 30 mA; and slits = 0.2 mm. Data processing utilized MacDiff software (Petschick, 2001). Profiles were smoothed using the MacDiff standard weighted means 17-term filter. To correct for misalignments of the goniometer and slide holder, we shifted each diffractogram to realign the quartz (100) reflection at 4.26 Å.
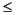 50% illite, and R = 1 ordering typically occurs at 55%-60% illite. The development of ordering may be recognized by a shift of the second-order I/S superstructure from ~5° to 6.5°2
50% illite, and R = 1 ordering typically occurs at 55%-60% illite. The development of ordering may be recognized by a shift of the second-order I/S superstructure from ~5° to 6.5°2