- Mg2+ +
2CaCO3
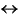 CaMg(CO3)2 + Ca2+.
CaMg(CO3)2 + Ca2+.
Concentrations of volatile hydrocarbon gases were measured from every core using the standard ODP headspace sampling technique and gas chromatographic analysis. Methane only occurred in very minor concentrations (1 to ~2.5 ppmv) (Table T8).
The low gas content at Site 1194 is likely a function of two characteristics of the sediment: appreciable pore water SO42- concentrations are likely to be limiting methanogenesis, and the organic matter that does exist is immature relative to hydrocarbon expulsion and petroleum generation.
Pore water samples in Hole 1194A were taken approximately every 10 m down to 132.6 mbsf. Sampling continued more intermittently to a depth of 418.0 mbsf in Hole 1194B, where cemented carbonate intervals and reduced recovery limited collection of pore waters (Fig. F32; Table T9). In some samples from Hole 1194B, it was not possible to collect sufficient water from the sediments to complete all analyses.
Pore water chloride increases from a value of 558 mM at 2.9 mbsf to 567 mM at 20.1 mbsf (Fig. F32A). From this depth to 300 mbsf, chloride concentration ranges narrowly, between 565 and 563 mM. Values are somewhat more variable through the remainder of the cored interval.
Alkalinity shows a slight increase, from 2 to 2.9 mM, in the upper 30 mbsf, then decreases in a linear fashion to ~0.68 mM at a depth of 254.9 mbsf and remains at that level to the bottom of the hole (Fig. F32B). The low alkalinity is unusual when considered together with significant bacterial sulfate reduction (discussed below). The 8 mol of sulfate reduction that occur in the upper 130 mbsf should add 16 mol of alkalinity to the pore fluids. This "missing" alkalinity is most likely precipitated as a carbonate mineral.
Sulfate concentration decreases steadily through the upper 120 mbsf, from 28.85 mM in the shallowest sample to ~18 mM within lithologic Unit IV (Fig. F32C) and remains relatively constant at 18 mM to the bottom of the cored section. The decrease in sulfate is likely due to bacterial sulfate reduction, with the modest removal of sulfate attributable to the low organic carbon content of the sediments (see "Sedimentary Geochemistry").
Associated with the reoxidation of sedimentary organic matter via sulfate reduction is the release of ammonium to the pore waters. Thus, the ammonium profile mirrors the sulfate profile (Fig. F32C, F32D). Ammonium concentration increases steadily over the upper 130 m of the sediments, reaching 600 µM near 340 mbsf, the depth where sulfate reduction ceases.
In lithologic Units I and II, the magnesium concentration decreases almost linearly from 54.5 mM at the surface to 48.26 mM at 132.6 mbsf (Fig. F32E). Below that depth, magnesium concentration remains relatively constant to ~300 mbsf then decreases to 34 mM at the base of Hole 1194B. The pore water calcium concentration increases in the uppermost 130 mbsf from 12.5 to 21.1 mM (Fig. F32F). Below 189 mbsf, the calcium concentration increases more rapidly with depth, reaching ~64 mM at 419 mbsf, the base of Hole 1194B.
In Hole 1194A, the concomitant decrease in pore water magnesium and increase in calcium concentration could be taken as evidence of minor dolomite formation by the reaction
Although the concentrations are linearly correlated (Fig. F33), the slope of the relationship is not as would be expected from this reaction. As at Site 1192, magnesium and sulfate are highly correlated with a slope close to one (Fig. F34), suggesting dolomite formation via the reaction
with the necessary 2 mol of alkalinity supplied by bacterial sulfate reduction. Pore water alkalinity values support dolomite formation by this second reaction, which also explains the missing alkalinity sink.
The changes in pore water calcium and magnesium seen below 180 mbsf (Fig. F32E, F32F) in the more lithified lithologic Units IV and V are not likely to be the result of dolomite formation, as the concentration of the two ions does not show a 1:1 correlation (Fig. F33). It is more likely that the observed profiles result from alteration of mafic basement rocks and subsequent diffusive exchange with the pore fluids in the overlying sediments. Some combination of diffusion and further minor dolomitization of the sediments, however, cannot be ruled out.
Pore water strontium concentration increases nearly linearly through lithologic Units I and II, rising from 105 µM at 2.9 mbsf to ~580 µM at 132 mbsf (Fig. F32G). Calcite recrystallization in this interval releases strontium to the pore water. The lack of further increase with depth suggests either no further carbonate recrystallization or that the strontium concentration is limited by celestite solubility. The relatively high sulfate concentration would titrate strontium from the pore waters as it is released by recrystallization. Significant carbonate recrystallization, however, should eventually led to a pattern of decreasing sulfate concentration and increasing strontium concentration, which is not observed. Potassium concentration decreases nearly linearly from 11.13 mM at 10.6 mbsf to values near 3.5 mM at 360 mbsf after a slight increase in the uppermost 10 m of the hole (Fig. F32H). Below 360 mbsf, concentrations remain relatively constant.
Ninety-one sediment samples were analyzed for carbonate mineralogy from Site 1194. Data are presented in Figure F35 and Table T10. The hemipelagic sediments of lithologic Units I and II are composed almost entirely of calcite, with a few samples containing 2-3 wt% dolomite. Aragonite was not detected. The hardground at the transition to lithologic Unit III contains ~50 wt% dolomite. Lithologic Subunit IIIA averages ~20 wt% dolomite, whereas Subunit IIIB is nearly pure calcite. In lithologic Units IV and V, dolomite is consistently ~10 wt% except near the base of Unit V where the sediments are pure calcite.
Calcium carbonate (CaCO3) content at Site 1194 ranges from ~45 to 99 wt% (Fig. F36; Table T11). The total organic carbon (TOC) content of all samples measured at Site 1194 is <0.5 wt%, with the highest values existing in the uppermost 100 m of the sediment section. Note that TOC content from Rock-Eval pyrolysis and carbon-nitrogen-sulfur analyses provide similar decreasing downsection profiles (Fig. F36; Tables T11, T12) and that TOC values covary inversely with CaCO3 content (Fig. F36).
Hydrogen index (HI) values at Site 1194 range from 29 to 300 mg HC/g TOC (Fig. F36; Table T12). Oxygen index (OI) values vary from 0 to 45,400 mg CO2/g TOC (Table T12). The high OI values measured are attributed to the thermal degradation of calcium carbonate during pyrolysis and are not considered in this interpretation. The low TOC values in some intervals at Site 1194 limit the reliability of some HI values. We performed duplicate and triplicate analyses on these samples, which indicated a reproducibility of ±10%. Tmax values ranging from 368° to 422°C (Table T12), although the most reliable Tmax values cluster between 400° and 420°C. Total S content in Site 1194 sediments range from 0 to ~0.67 wt% (Fig. F36; Table T11).
Variations in the generally high calcium carbonate content (average = ~82 wt%) (Fig. F36) of sediments at Site 1194 mostly reflect fluctuations in the ratio of biogenic carbonate and terrigenous sedimentation to the seafloor through time. Calcium carbonate content exhibits an overall increase from 89 wt% at 2.2 mbsf to 95 wt% at 118 mbsf. This interval corresponds to lithologic Unit II (see "Lithostratigraphy and Sedimentology") and contains well-preserved foraminifers, and lesser amounts of calcareous nannofossils (see "Biostratigraphy and Paleoenvironments"). The noncarbonate sediment in this interval is primarily clay, with between ~0.2 and 0.5 wt% TOC. Firmgrounds clustered between ~34 and 42 mbsf (see "Lithostratigraphy and Sedimentology") appear to correspond to a slight decrease in calcium carbonate content (~59 and ~70 wt%, respectively) and elevated percent TOC values (0.48 and 0.42 wt%, respectively) obtained from Rock-Eval pyrolysis. The relatively elevated TOC content with mostly low HI values and several extremely high C/N ratios likely indicate the presence of terrigenous organic matter. The very high C/N values may represent the presence of inertinite within the terrigenous debris. These interpretations are at least partially corroborated by observations of wood fragments at ~10-15 and ~110-115 mbsf (see "Lithostratigraphy and Sedimentology") and fusain in a number of the samples described in this section. The relatively high total S content in this interval (Fig. F36) and observations of pyrite (see "Lithostratigraphy and Sedimentology") suggest that pyrite formation was influenced by increased organic matter influx to the seafloor.
Sediments from ~118 to 300 mbsf can be organized into three geochemically defined units. Each unit consists of a conspicuous horizon of increased percent TOC values, low HI values, and relatively elevated percent total S content, overlying a broader interval of relatively high CaCO3, low TOC, and higher HI values. The uppermost horizon appears to coincide with a firmground, and the uppermost unit, ranging from ~117 to 170 mbsf, corresponds to lithologic Unit III (see "Lithostratigraphy and Sedimentology"). A slight decrease in carbonate content between ~150 and 160 mbsf corresponds to the Subunit IIIA/IIIB boundary, an inferred hardground (see "Lithostratigraphy and Sedimentology") and a prominent seismic reflection (see "Seismic Stratigraphy").
The most prominent geochemical horizon exists between ~177 and 187 mbsf at Site 1194, marked by the lowest CaCO3 content (45%) observed at the site, and appears to coincide with a second inferred firmground and the boundary between lithologic Units III and IV (see "Lithostratigraphy and Sedimentology"). It overlies a unit of relatively elevated carbonate and depressed TOC content from ~190 to 225 mbsf. The third prominent horizon at ~235 mbsf appears to correspond to a firmground (see "Site 1194 Visual Core Descriptions") and again overlies an interval of elevated carbonate and HI values and depressed TOC content to a depth of ~295 mbsf. This horizon corresponds to a peak in the natural gamma ray log (see "Downhole Measurements") and is interpreted as part of the deepest depositional environment recorded at Site 1194 (see "Biostratigraphy and Paleoenvironments").
Calcium carbonate content decreases to ~61 wt% in the interval ~295-313 mbsf and then displays an overall increase to ~92 wt% toward the bottom of the hole. Through this same interval, TOC values increase to ~0.3 wt% at ~324 mbsf and then decrease to <0.1 wt% at total depth; sulfur values are depressed relative to samples higher in the section. This interval roughly corresponds to the lower portion of lithologic Unit IV (see "Lithostratigraphy and Sedimentology"). Below the percent TOC peak at ~324 mbsf, carbonate and TOC values covary inversely; this interval roughly corresponds to lithologic Subunits VA and VB (see "Lithostratigraphy and Sedimentology").