METHODS
Cores from Site 1207 were reexamined and described (Fig. F5) at the ODP Gulf Coast Core Repository at Texas A&M University (USA) in order to verify color trends from original shipboard descriptions (Fig. F2). It is not known if the original shipboard descriptions were based on wet vs. dry chert or whether all visible chert colors or only the predominant chert colors were recorded. Chert was wetted prior to description, and color was classified using a Geological Society of America (GSA) rock color chart (Rock-Color Chart Committee, 1991). Sampling was limited to the base of the section (Barremian) at this site (Fig. F6), an interval not cored at Sites 1213 and 1214.
A total of 95 sample chips were collected from core recovered during Leg 198, mainly from Site 1213, but additional samples were collected from Sites 1207 and 1214 (Table T2). Sample intervals were chosen where stratigraphy was most intact, as well as where a variety of lithologies were present. Whenever possible, at least one sample of chalk, porcellanite, and chert was taken per core, and if different colors of chert were present, each major color was sampled. All sample chips were described in terms of color, composition, and sedimentary structures using a hand lens, stereoscopic microscope, and GSA color chart.
Once described, 55 of the 95 chosen samples of chert, porcellanite, and chalk/limestone were selected for thin section preparation (Fig. F6; Table T3). These samples were split (cut), and a subsample was impregnated with blue-dyed epoxy, which highlights microporosity distribution, prior to thin section production. The 55 thin sections were described using a petrographic microscope for composition, mineralogy, texture, lithology, and chert color, as well as visual estimations of percentages of components (Table T3).
Hand-specimen and thin-section descriptions were used to select representative samples of chert, porcellanite, and chalk/limestone for geochemical analyses using X-ray fluorescence (XRF) at Washington State University GeoAnalytical Laboratory (USA). Sample selection was based on volume, uniformity of lithology, availability of other nearby lithologies for comparison, age of units, and chert color. A total of 28 samples were chosen from Site 1213, and two were chosen from the base of Site 1207 (Fig. F6; Table T3). This set of samples includes 20 cherts (four red, nine brown, six gray, and one green), two porcellanitic cherts, one porcellanite, one siliceous limestone, and five limestones (lithologies based on X-ray diffraction [XRD] data). Samples were analyzed for major and trace elements, including Si, Al, Ti, Fe, Mn, Ca, Mg, K, Na, P, Ni, Cr, Sc, V, Ba, Rb, Sr, Zr, Y, Nb, Ga, Cu, Zn, Pb, La, Ce, and Th (Table T4). This is done by measuring and comparing the X-ray intensity for each element with the intensity for two beads each of nine US Geological Survey standard samples and two beads of pure vein quartz (used as blanks for all elements except Si). The 20 standard beads are run and used for recalibration approximately once every 3 weeks or after the analysis of ~300 unknowns. Intensities for all elements are corrected automatically for line interference and absorption effects. For each element, the limit of determination, the 2- uncertainty in precision, is reported (Table T4). Normalized data for each element, major and trace, was analyzed to determine similarity among varying rock types and colors. Loss on ignition (LOI), SO3, and Cl values (found using QuantAs semiquantitative scans) were also determined for most samples. SO3 and Cl were present in small to moderate amounts in some samples. Iron (II) vs. iron (III) was not differentiated, as the XRF technique can only detect iron atoms, not the valence state. No other elements, with the exception of W and Co (which are present at a few hundred ppm because of grinding bowl contamination), were detected.
uncertainty in precision, is reported (Table T4). Normalized data for each element, major and trace, was analyzed to determine similarity among varying rock types and colors. Loss on ignition (LOI), SO3, and Cl values (found using QuantAs semiquantitative scans) were also determined for most samples. SO3 and Cl were present in small to moderate amounts in some samples. Iron (II) vs. iron (III) was not differentiated, as the XRF technique can only detect iron atoms, not the valence state. No other elements, with the exception of W and Co (which are present at a few hundred ppm because of grinding bowl contamination), were detected.
Several of the samples total <98 wt% and, according to the criteria of Ragland (1989) for igneous rocks, are not acceptable. They are generally samples with high CaO contents and correspondingly high LOI values, essentially limestones and calcareous lithologies as verified by thin section and XRD analyses. The low weight percent totals could be due to several factors, including the presence of elements not analyzed. LOI was determined at 750°C, a temperature that might not have completely calcined carbonate minerals present. Some of the missing weight percent could also be associated with sulfur. Note that no distinct barite or gypsum/anhydrite peaks were noted in the XRD patterns (see below), but minor pyrite is present. The data were normalized (scaled proportionately) to 100% in order to compensate for these unanalyzed elements (e.g., C and S); this allows for sample comparison.
Powder diffraction data were collected on an Oxford Diffraction Xcalibur3 diffractometer equipped with a molybdenum X-ray source (2.0 kW; 1.00-mm beam diameter) and a Saphire3 charge-coupled device detector. Samples powdered using a small microdrill were packed into X-ray quality 0.70 mm glass capillary tubes that were then sealed and affixed to a brass pin. The samples were mounted on a Huber goniometer head and aligned in the X-ray beam. The data collection procedure was as follows: with the detector set 50 mm from the sample, a 5-min measurement of the CCD dark current was performed followed by two 5-min data collections over a 360° 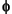 rotation of the sample. The two images were compared to remove any anomalous signals and then integrated and converted to a two-dimensional powder pattern using the Crysalis software package (www.oxford-diffraction.com). The data were exported to Microsoft Excel for analysis. A blank scan was obtained by mounting an empty capillary tube and collecting a data set using identical conditions. Baseline corrections on the samples were performed by a simple point by point subtraction of a scaled value of the blank measurement. The scale factor was chosen to provide as flat a baseline as possible while maintaining an overall positive intensity measurement in the corrected data set. Peak values were compared to known mineral diffraction data (e.g., silica minerals [
rotation of the sample. The two images were compared to remove any anomalous signals and then integrated and converted to a two-dimensional powder pattern using the Crysalis software package (www.oxford-diffraction.com). The data were exported to Microsoft Excel for analysis. A blank scan was obtained by mounting an empty capillary tube and collecting a data set using identical conditions. Baseline corrections on the samples were performed by a simple point by point subtraction of a scaled value of the blank measurement. The scale factor was chosen to provide as flat a baseline as possible while maintaining an overall positive intensity measurement in the corrected data set. Peak values were compared to known mineral diffraction data (e.g., silica minerals [ -quartz, cristobalite, and tridymite], carbonate minerals, etc.).
-quartz, cristobalite, and tridymite], carbonate minerals, etc.).

 uncertainty in precision, is reported (Table T4). Normalized data for each element, major and trace, was analyzed to determine similarity among varying rock types and colors. Loss on ignition (LOI), SO3, and Cl values (found using QuantAs semiquantitative scans) were also determined for most samples. SO3 and Cl were present in small to moderate amounts in some samples. Iron (II) vs. iron (III) was not differentiated, as the XRF technique can only detect iron atoms, not the valence state. No other elements, with the exception of W and Co (which are present at a few hundred ppm because of grinding bowl contamination), were detected.
uncertainty in precision, is reported (Table T4). Normalized data for each element, major and trace, was analyzed to determine similarity among varying rock types and colors. Loss on ignition (LOI), SO3, and Cl values (found using QuantAs semiquantitative scans) were also determined for most samples. SO3 and Cl were present in small to moderate amounts in some samples. Iron (II) vs. iron (III) was not differentiated, as the XRF technique can only detect iron atoms, not the valence state. No other elements, with the exception of W and Co (which are present at a few hundred ppm because of grinding bowl contamination), were detected.
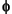 rotation of the sample. The two images were compared to remove any anomalous signals and then integrated and converted to a two-dimensional powder pattern using the Crysalis software package (
rotation of the sample. The two images were compared to remove any anomalous signals and then integrated and converted to a two-dimensional powder pattern using the Crysalis software package ( -quartz, cristobalite, and tridymite], carbonate minerals, etc.).
-quartz, cristobalite, and tridymite], carbonate minerals, etc.).