 H2S = H2S + HS-) dissolved in interstitial water is below the analytical detection limit of 0.2 µM at all depths sampled (Fig. F5H).
H2S = H2S + HS-) dissolved in interstitial water is below the analytical detection limit of 0.2 µM at all depths sampled (Fig. F5H).
Site 1225 is characterized by low organic carbon content in sediment and active fluid flow in the underlying basement (Baker et al., 1991). The interstitial water (IW) sampling strategy at this site was designed to clearly define biogeochemical zones and to allow for modeling biogeochemical reaction rates and associated chemical fluxes. Accordingly, IW samples were collected to coincide with samples for microbiological analysis and to concentrate on intervals where higher microbial activity was expected. The chemical data set includes shipboard analyses not routinely employed during ODP cruises (hydrogen, acetate, formate, nitrate, dissolved oxygen, DIC, and high-resolution manganese and iron) as well as quantitative analyses of methane at trace levels.
A total of 29 interstitial water samples were taken from Hole 1225A, one from each of the upper three cores, one from every other core starting with Core 201-1225A-4H (except for Core 18H), and seven additional samples at various intervals (Table T2). The upper two cores were sampled extensively in Hole 1225C, with at least one sample taken from every section of Core 201-1225C-1H and three samples from Core 2H. At greater depths, two interstitial water samples were collected from each core until drilling was terminated at a depth of 305.3 mbsf.
Overall, microbially mediated reactions drive the variations in interstitial water composition. These reactions result in distinct biogeochemical zones; their establishment and maintenance is largely controlled by diffusive processes across the sediment/water and sediment/basement interfaces as well as by reactions with solid phases in the sediment. Moving from the interfaces toward the sediment in between, we observed a sequence of biogeochemical oxidation-reduction zones, including zones of aerobic respiration, nitrate reduction, manganese reduction, iron reduction, and, finally, sulfate reduction.
Alkalinity and DIC have similar downhole profiles. Alkalinity increases from a near-surface value of 2.92 mM to a maximum of 4.25 mM at depths between 100 and 200 mbsf, followed by a decrease downhole (Fig. F5A). There is also a local minimum centered near 50 mbsf, indicating local alkalinity consumption. DIC ranges from a low of 3.2 mM at 3 mbsf to a high of 4.0 mM at 201.7 mbsf; at greater depths DIC decreases to ~3.2 mM at the bottom of Hole 1225C (Fig. F5B). These are the first high-resolution DIC measurements obtained during an ODP leg. Technique development at this first site undoubtedly contributed to the variability in the data, especially in the earliest measurements. DIC is produced by microbial respiration, and alkalinity is primarily produced during sulfate reduction. The net changes in DIC and alkalinity result from production as well as carbonate precipitation driven by equilibration. As with many of the dissolved species at Site 1225, the decrease toward seawater concentrations at depth is due to exchange with water in the underlying basaltic basement.
Dissolved oxygen measurements were conducted by microelectrode on selected core sections from Hole 1225C (Sections 201-1225C-1H-1, 1H-2, 1H-3, 2H-1, 2H-2, 2H-5, 32H-7, 33H-2, and 33H-6) and Hole 1225A (Section 201-1225A-35X-5). In each sample, except the one from Section 201-1225A-35X-5, measurements were conducted by inserting the microelectrode probe and reference directly into the core through holes drilled into the core liner. For the core sample from Section 201-1225A-35X-5, the electrodes were inserted into a segment of the IW whole-round core after loose material had been scraped away. Three sections had detectable concentrations of oxygen: the bottom of Section 201-1225C-1H-1 (50 µM), the top of Section 1H-2 (30 µM), and Section 201-1225A-35X-5 (50 µM). Based on uncertainty in the blank and measured potentials, the analytical uncertainty is estimated to be ~30% for these measurements. The possibility of oxygen infiltrating the sample as a result of cracking associated with the insertion of the probe adds additional uncertainty to the measured oxygen concentration for Section 201-1225A-35X-5; however, accepting the reliability of this measurement implies that the fluid circulating in the underlying basement is oxic.
Nitrate concentrations decrease from 33 µM near the sediment/water interface to <1.5 µM at 1.5 mbsf (Fig. F5C). Values remain indistinguishable from zero until ~12 m above the sediment/basement interface and increase to 23 µM in the deepest sample collected (Section 201-1225A-35X-5). Both the shallow and deep gradients are due to microbial nitrate reduction balanced by diffusion into the sediment column. The gradient at the bottom of the hole is consistent with diffuse exchange with a basement fluid that has a nitrate concentration close to that of modern Pacific bottom water (~36 µM) (Levitus et al., 1994).
The downhole profile of dissolved manganese concentrations shows five general features (Fig. F5D). Values increase quasi-linearly from the shallowest sample to a peak of 158 µM at 3.65 mbsf (Sample 201-1225C-1H-3, 65-80 cm). Concentrations then decline steadily to ~4 µM at a depth of 110 mbsf and remain below 4 µM, locally decreasing to 2 µM, until 210 mbsf. Dissolved manganese then increases to form a broad peak with values exceeding 7 µM at 250 mbsf. Concentrations are <4 µM from 290 mbsf to the bottom of the hole.
Dissolved manganese has been measured previously at numerous ODP sites, although never in as much detail as at Site 1225. The general profile at this site is similar to that at other open-ocean sites having low to moderate primary productivity in surface waters (e.g., Site 881, northwest Pacific [Shipboard Scientific Party, 1993], and Site 999, Caribbean Sea [Shipboard Scientific Party, 1997]). The concentration peak at 3.65 mbsf probably results from the dissolution of manganese oxide phases driven by microbial manganese reduction. The change in gradient at ~100 mbsf is due to manganese precipitation, most likely as a sulfide phase. The deeper maximum in manganese at 250 mbsf may again reflect bacterial reduction of solid manganese oxide phases.
The downhole profile of dissolved iron concentrations shows considerably more scatter than the dissolved manganese data (Fig. F5E). The overall profile consists of two broad peaks centered at ~25 and 230 mbsf. The two iron peaks thus occur more distant from the surface and basement interfaces than the manganese peaks and coincide with intervals of relatively high magnetic susceptibility (see Fig. F17). The dissolved iron maxima likely represent release of iron from solid phases such as magnetite by bacterial iron reduction.
The ~50% variation in the iron concentrations of adjacent interstitial water samples raises an intriguing issue because such steep concentration gradients cannot be maintained over significant time. The scatter does not reflect analytical imprecision, which is within ±5% as determined by repeated analysis of samples. Rather, the scatter likely reflects either (1) oxidation during squeezing or water handling that has removed dissolved iron in some samples or (2) detection of particulate and colloidal iron with particle sizes smaller than the pore size of the filter (0.45 µm) used in collection of the interstitial waters.
Dissolved sulfate concentrations decrease from 28.8 mM at 1.5 mbsf to 27 mM at 83 mbsf and remain near that value for the remainder of the hole (Fig. F5F).
The downhole profile of dissolved strontium is similar to that obtained for Site 851 (Shipboard Scientific Party, 1992). Concentrations are similar to seawater (86 µM) in the uppermost sample at 0.37 mbsf and then steadily rise to 170-186 µM between 220 and 270 mbsf (Fig. F5G). Concentrations sharply decrease to near seawater values above basement. The overall profile suggests an active zone of carbonate recrystallization in lithostratigraphic Subunits ID and IE, with diffusive exchange with a basement fluid similar to seawater.
Concentrations of barium and selected transition metals (copper, molybdenum, nickel, vanadium, and zinc) were below their detection limits in all samples analyzed from Site 1225.
Total sulfide ( H2S = H2S + HS-) dissolved in interstitial water is below the analytical detection limit of 0.2 µM at all depths sampled (Fig. F5H).
H2S = H2S + HS-) dissolved in interstitial water is below the analytical detection limit of 0.2 µM at all depths sampled (Fig. F5H).
The volatile fatty acids, acetate and formate, were examined in 22 IW samples from Holes 1225A and 1225C (Table T2; Fig. F5I). In the majority of samples, acetate was below the detection limit of ~0.5 µM. Formate concentrations exceeded the detection limit of ~0.2 µM in one-half of the samples, with the maximum concentration of 0.9 µM present in the shallowest sample at a depth of 1.50 mbsf (Sample 201-1225A-1H-2, 0-15 cm). Undetectable or extremely low concentrations of acetate distinguish Site 1225 from nearshore coastal environments, where acetate concentrations of ~10 µM are typical.
Methane gas analyses performed during Leg 138 suggested that this gas is present in very low concentrations, presumably at submicromolar levels, throughout the sediment column (Mayer, Pisias, Janecek, et al., 1992). To examine these low gas concentrations, two series of headspace gas analyses were conducted in parallel (see "Gas Analyses" in "Biogeochemistry" in the "Explanatory Notes" chapter): (1) the standard procedure in fulfillment of the hydrocarbon monitoring program for safety purposes and (2) a modification of the latter procedure aimed at a more accurate determination of methane concentrations in interstitial water.
Methane was detected in all samples from Holes 1225A and 1225C, with the exception of samples from the very top and bottom of the respective holes. No other hydrocarbons were detected. Methane yields in headspace gas depended on the protocol employed. Methane concentrations in headspace were highest in samples that were extracted for 24 hr and lowest in samples prepared according to the standard safety protocol (extracted at 60°C for 20 min) (Table T3; Fig. F6A). Note that differences in headspace methane between Holes 1225A and 1225C are attributed to the extended extraction time utilized for samples from the latter hole rather than to differences in concentration in these two adjacent holes. Independent of the analytical protocol, all measured values are <3 ppm in the headspace. Even though analyses of methane concentrations in ambient air were highly precise with a standard deviation ( ) below ±0.5 ppm per volume headspace, replicate analyses of selected closely spaced sediment samples had a range approximately twice as large. Accordingly, the relatively high variance of data on a vertical scale of meters to a few tens of meters is interpreted to result from analytical conditions.
) below ±0.5 ppm per volume headspace, replicate analyses of selected closely spaced sediment samples had a range approximately twice as large. Accordingly, the relatively high variance of data on a vertical scale of meters to a few tens of meters is interpreted to result from analytical conditions.
Concentrations of methane in interstitial waters were calculated for both Holes 1225A and 1225C using the porosity estimates derived from gamma ray attenuation (GRA) densitometer measurements (see "Density and Porosity" in "Physical Properties"). Despite the variance related to analytical conditions, the overall distribution of methane is well defined. Concentrations increase from background to a broad maximum of 0.1 to 0.2 µM between ~100 and 250 mbsf (Table T3; Fig. F6B). Methane concentrations return to background levels at depth, with the gradient toward the basement being somewhat steeper than the gradient at the sediment/water interface. The broad maximum may reflect a thermodynamic equilibrium concentration under conditions of high sulfate concentrations.
Incubations for hydrogen were conducted on 16 samples. All samples were incubated at 4°C, the approximate midrange temperature (range = 1.4°-7.0°C) of the sediment column (see "Thermal Conductivity" in "Physical Properties"). Calculated interstitial fluid concentrations of the incubated sediments are shown in Table T4 and Figure F5J. Between the shallowest hydrogen sample (7.45 mbsf) and 121.45 mbsf, there is a generally monotonic increase from 0.6 to 2.0 nM, except for one sample at a depth of 45.45 mbsf, which has a significantly higher concentration of 3.0 nM. From 140.45 mbsf to the deepest sample at 307.3 mbsf, hydrogen concentrations are lower, ranging from 0.6 to 1.4 nM (average = 1.0 nM). It has been previously observed that hydrogen concentrations measured by this method are defined by the predominant electron acceptor (Lovley and Goodwin, 1988; Hoehler et al., 1998). Concentrations are similar to those observed by Hoehler et al. (1998) in shallow nearshore marine sediments and in experiments where sulfate reduction occurs. A more detailed consideration of the controls on hydrogen concentration requires the analysis of free energies of the dissimilatory reactions that involve hydrogen and are based on measured concentrations of reaction products as well as reactants.
In Hole 1225A, interstitial water ammonium concentrations are low in the top and bottom sections of the hole, with minima 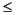 6 µM (Fig. F5K). A broad maximum of 70-77 µM is present at 125-200 mbsf. A similar profile with a broad maximum in ammonium concentrations of ~90-101 µM at the same depth interval was observed previously in Hole 851B (Shipboard Scientific Party, 1992). The deep maximum indicates that production of ammonium.
6 µM (Fig. F5K). A broad maximum of 70-77 µM is present at 125-200 mbsf. A similar profile with a broad maximum in ammonium concentrations of ~90-101 µM at the same depth interval was observed previously in Hole 851B (Shipboard Scientific Party, 1992). The deep maximum indicates that production of ammonium.
Dissolved phosphate decreases from 4 µM, a value slightly above local bottom-water concentration, to ~2 µM between 7 and 16 mbsf (Fig. F5L). It is then essentially invariant to a depth of at least 200 mbsf, with the exception of a slight increase from 25 to 75 mbsf. The steep gradient at shallow depths to a minimum at 16 mbsf indicates precipitation of a phosphatic phase such as apatite. The low, relatively invariant phosphate concentrations as compared to ammonium at greater depths suggest that dissolved phosphate concentrations are controlled by equilibrium with a solid phase.
Dissolved silica concentrations increase from 650 µM at the seafloor to 1050 µM at ~180 mbsf (Fig. F5M). The concentrations then vary between 950 and 1100 µM to the base of the hole. The overall downhole trend reflects net dissolution of biogenic silica until a depth of ~150 mbsf. The scatter likely reflects a combination of analytical precision (±40 µM) and variable silica content. Interestingly, silica concentrations do not decrease at the base of the hole, unlike those reported for Site 851 (Shipboard Scientific Party, 1992).
Chloride concentrations increase from 555.1 mM near the sediment/water interface to a maximum of 565.9 mM at a depth of 27.8 mbsf (Fig. F5N). This trend is most likely due to the diffusion of chloride from high-chlorinity glacial seawater out of the sediment column (McDuff, 1985). The values then decline to 563.4 mM at 308 mbsf. This downhole decrease may result from the release of water associated with amorphous silica diagenesis and/or the transport of lower-salinity water from the basement.
An extensive set of inorganic carbon analyses exists for Site 851; consequently, during Leg 201 only a broad overview was obtained to provide data for microbiological relationships. The inorganic carbon results from Site 1225 are comparable to those obtained from Site 851 (Shipboard Scientific Party, 1992). In the upper 220 mbsf, CaCO3 percentages are high and variable, ranging from 57% to >80%, with most values between 70% and 80% (Table T5; Fig. F7). Two lower values (42%) are present between 266 and 270 mbsf, with higher values again present at greater depths. The lower CaCO3 percentages at both Site 851 and Site 1225 correspond to more siliceous intervals in the cores (see "Lithostratigraphy").
Total organic carbon (TOC) concentrations are low throughout the section, in agreement with the results from Site 851 (Table T5; Fig. F7). All values for TOC are 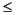 0.42%, with most values falling
0.42%, with most values falling 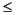 0.2%.
0.2%.