GEOCHEMISTRY
Concentrations of headspace gases were routinely monitored in Hole 1237B sediments according to shipboard safety and pollution prevention considerations. Little gas was found at Site 1237 (Fig. F33; Table T17). Very low amounts of methane were detected in the shallowest headspace gas sample at 4.5 mcd. Below this depth, methane gradually increased, reaching a maximum of 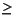 7 ppmv between 33.3 and 63.1 mcd. Then methane concentrations decreased to concentrations near the laboratory air concentrations (~2-3 ppmv at 95.3 mcd) and remained at this level to the bottom of the hole. The interval where methane is detected corresponds to the interval where significant concentrations of organic matter were measured (see "Sedimentary Inorganic Carbon, Organic Carbon, and Nitrogen Concentrations"). At depths
7 ppmv between 33.3 and 63.1 mcd. Then methane concentrations decreased to concentrations near the laboratory air concentrations (~2-3 ppmv at 95.3 mcd) and remained at this level to the bottom of the hole. The interval where methane is detected corresponds to the interval where significant concentrations of organic matter were measured (see "Sedimentary Inorganic Carbon, Organic Carbon, and Nitrogen Concentrations"). At depths 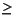 95 mcd, both methane and organic carbon are near detection limits. Methane probably originates from in situ fermentation (methanogenesis) of the organic matter buried in the sediments (Claypool and Kvenvolden, 1983). In addition, the low amounts of methane together with the high organic matter contents suggest that only a small amount of organic material is converted to gas. No higher molecular weight hydrocarbons were detected.
95 mcd, both methane and organic carbon are near detection limits. Methane probably originates from in situ fermentation (methanogenesis) of the organic matter buried in the sediments (Claypool and Kvenvolden, 1983). In addition, the low amounts of methane together with the high organic matter contents suggest that only a small amount of organic material is converted to gas. No higher molecular weight hydrocarbons were detected.
Interstitial Water Geochemistry
We collected 35 interstitial water samples from Site 1237, 1 from Hole 1237A and 34 from Hole 1237B. These are treated as constituting a single mcd profile. Chemical gradients at this site (Table T18; Fig. F34) reflect minor influence in the uppermost sediments of organic matter diagenesis by microbially mediated oxidation reactions, a limited degree of biogenic opal dissolution, and a minor signature of calcite diagenesis. For many elements, interstitial water geochemistry does not vary with lithologic unit (see "Description of Lithologic
Units" in "Lithostratigraphy") or with the significant color change observed at ~170 mcd (Fig. F34).
Chlorinity generally increases with depth to 566 mM at 320.9 mcd, with slightly lower values in the deepest three samples (Fig. F34). Salinity, measured refractively as total dissolved solids, ranges from 34 to 36 (Table T18). Sodium concentrations measured by inductively coupled plasma-atomic emission spectrophotometry averaged 2% lower than those estimated by charge balance reported here (Table T18). Sodium concentrations parallel chlorinity, with a total range from 482 to 490 mM.
Organic matter diagenesis, driven by microbial oxidation reactions, has a relatively minor influence on interstitial water composition. Sulfate undergoes a limited degree of reduction primarily in the uppermost sediments, by no more than 4 mM from typical seawater values of ~29 mM. Alkalinity has a shallow maximum of >5 mM from 9.1 to 20.6 mcd, declines to <3.5 mM by the Unit I/II boundary, and then declines more slowly to ~2.2 mM from 332.7 to 344.3 mcd.
Dissolved manganese decreases rapidly from 5.4 然 at 4.5 mcd to low values in Subunit IB and persists at low values (near or below the detection limit of 0.1 然) throughout the drilled interval. The marked color change at ~170 mcd has no corresponding signature in manganese redox chemistry in interstitial water. Dissolved iron is generally below the detection limit (<1.1 然), reflecting the low content of terrigenous material in this site.
Phosphate concentrations decrease steeply in Subunit IA and more slowly in Subunit IB, from 8.9 然 at 4.5 mcd to <2 然 throughout Unit II. Ammonium concentrations increase from 220 然 at 4.5 mcd to peak values >250 然 from 9.1 to 33.3 mcd, then decline in Subunits IB and IIA to values near or below the detection limit (11 然) in Subunit IIB. The shallow peaks in alkalinity, manganese, phosphate, and ammonium, along with minor sulfate reduction, result from degradation of organic matter in the upper ~100 mcd.
Dissolved silicate has highest values in the depth range where diatoms are reported to have the best preservation and abundance (see "Diatoms" in
"Biostratigraphy"), averaging ~800 然 from 4.5 to 63.0 mcd. Silicate concentrations decline to 543 然 at 177.3 mcd and average 325 然 in the depth interval observed to be barren of diatoms (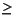 194.2 mcd). The interstitial waters are undersaturated with respect to biogenic opal (saturation value = >1000 然). Barium concentrations are below the detection limit (<0.03 然) throughout. Boron concentrations, initially greater than seawater concentrations, decrease with increasing depth, from >500 然 from 4.5 to 41.8 mcd to 450 然 at 355.0 mcd.
194.2 mcd). The interstitial waters are undersaturated with respect to biogenic opal (saturation value = >1000 然). Barium concentrations are below the detection limit (<0.03 然) throughout. Boron concentrations, initially greater than seawater concentrations, decrease with increasing depth, from >500 然 from 4.5 to 41.8 mcd to 450 然 at 355.0 mcd.
Calcium and magnesium concentrations increase slightly with increasing depth, with a larger relative increase in calcium resulting in decreasing magnesium/calcium ratios with depth from 5.3 at 4.5 mcd to 4.8 at 355.0 mcd (Fig. F34). Authigenic mineralization reactions have a limited influence on interstitial water geochemistry at this site, with no signatures of intense calcite precipitation or dolomite formation. Lithium concentrations decrease from 24 然 at 4.5 mcd to a broad minimum of <14 然 from 63.0 to 125.5 mcd, then increase again with depth to 26-27 然 from 275.9 to 355.0 mcd. Strontium concentrations are greater than seawater values in the shallowest samples, increasing to a maximum of >200 然 from 33.3 to 135.7 mcd. The strontium profile is similar in character, although with a smaller maximum value, to those in calcium carbonate-rich, equatorial sites, with the strontium maximum resulting from the recrystallization of biogenic calcite to authigenic calcite (e.g., Delaney et al., 1991). Potassium concentrations decrease slightly with depth (Fig. F34).
Sedimentary Inorganic Carbon, Organic Carbon,
and Nitrogen Concentrations
Inorganic carbon (IC), total carbon (TC), and total nitrogen (TN) concentrations were determined on sediment samples from Hole 1237B (Table T19). Organic matter carbon/nitrogen ratios and Rock-Eval pyrolysis were employed to characterize the organic matter.
Calcium carbonate concentrations range between 0.9 and 96.9 wt% (Table T19; Fig. F35). In samples from the uppermost 38 mcd, calcium carbonate contents are low, averaging 12.2 wt%, and vary between 1.0 and 37.6 wt%. Between 38 and ~80 mcd, calcium carbonate contents increase to ~90 wt%. From ~80 to 182 mcd, calcium carbonate contents vary between 70 and 96 wt%. Some intervals with lower calcium carbonate are likely due to the presence of volcanic ash (see "Description of Lithologic
Units" in "Lithostratigraphy"). From ~182 to 357 mcd, calcium carbonate contents are consistently >94 wt% (average = 95.7 wt%). A significant change in calcium carbonate characterizes the interval from 38 to 80 mcd and may reflect the combined influence of the tectonic backtrack path of the site toward more coastal conditions with a greater delivery of terrigenous material and biogenic silicate (opal) to the site (see Fig. F12).
TOC contents range between 0.3 and 1.9 wt% in the uppermost 38 mcd and from 0.4 wt% to below detection limit at greater depth (Table T19; Fig. F35). The two uppermost samples show the highest values. The TOC profile shows a gradual and long-term decrease from the top to values <0.1 wt% by 59.3 mcd, associated with large-amplitude variations of ~1 wt%, especially in the uppermost 40 mcd. Variations in TOC and calcium carbonate contents cannot be attributed to dilution alone, as the relationship between TOC and calcium carbonate is not linear (Fig. F36). Besides, these variations cannot be explained by a dissolution effect of carbonate resulting from organic matter degradation since microfossils are generally well preserved (see "Biostratigraphy"). The change in TOC concentrations is most likely the result of greater biological production resulting from enhanced nutrient supply via upwelling of nutrient-rich waters. This would be consistent with the tectonic backtrack path exposing Site 1237 to more coastal conditions during the last 5 m.y. In addition, the role of the Andean uplift by forcing coastal winds to blow parallel to the coastline may have been one of the forcing parameters allowing the establishment and/or the intensification of the coastal upwelling during this period. At greater depths, in the sediments where calcium carbonate contents are very high, total organic carbon content could not be determined with confidence by shipboard techniques and is probably <0.1-0.2 wt% (Romankevitch, 1984).
In order to characterize the organic matter in the sediments and to explain its steep gradient with depth, TOC/TN ratios and Rock-Eval results were used. Site 1237 TOC/TN ratios vary from 7 to <2 (Table T19; Fig. F35). The TOC/TN ratios average 4.9, a value which is typical of unaltered algal material (5-8) (Bordovskiy, 1965; Emerson and Hedges, 1988; Meyers, 1997). The TOC and TOC/TN changes are most likely driven by the change in the supply of terrigenous organic matter.
Ten samples that span the interval of the downhole decrease in TOC concentrations in the uppermost 26 mcd were selected for Rock-Eval measurements (Table T20). The low Tmax values indicate that organic matter is thermally immature. The relationship between S2 and TOC (Fig. F37) shows that the organic matter is between Type II (i.e., marine algal organic matter) and Type III (i.e., land-derived organic matter) (Tissot and Welte, 1984; Langford and Blanc-Valleron, 1990). This is not consistent with TOC/TN ratios that indicate that the organic matter is of marine origin. Fresh marine plankton has a relatively high lipid content and thus high H/C ratios. Therefore, well-preserved organic matter of marine algal origin yields high hydrogen index (HI) values when subjected to pyrolysis. The HI values measured in these samples, which range from 114 to 227 (Table T16), indicate that the organic matter is significantly degraded. A subtle change in the supply of land-derived organic matter cannot explain alone the HI and TOC/TN values. Therefore, one reason for the downhole decrease in TOC contents, together with TOC/TN ratios and HI values, could be an increase in the degradation of the organic matter with burial time. Another reason could be a downhole decrease in the preservation of the organic matter buried in the sediments because of a decrease in export productivity in the past. Such a change in export productivity is also suggested by a parallel change in diatom, benthic foraminifer, and organic matter abundance (see "Biostratigraphy"). This downhole change toward more eutrophic conditions would be consistent with the tectonic backtrack path of the site and may be consistent with the establishment or intensification of coastal upwelling due to Andean uplift.

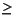 7 ppmv between 33.3 and 63.1 mcd. Then methane concentrations decreased to concentrations near the laboratory air concentrations (~2-3 ppmv at 95.3 mcd) and remained at this level to the bottom of the hole. The interval where methane is detected corresponds to the interval where significant concentrations of organic matter were measured (see "Sedimentary Inorganic Carbon, Organic Carbon, and Nitrogen Concentrations"). At depths
7 ppmv between 33.3 and 63.1 mcd. Then methane concentrations decreased to concentrations near the laboratory air concentrations (~2-3 ppmv at 95.3 mcd) and remained at this level to the bottom of the hole. The interval where methane is detected corresponds to the interval where significant concentrations of organic matter were measured (see "Sedimentary Inorganic Carbon, Organic Carbon, and Nitrogen Concentrations"). At depths  95 mcd, both methane and organic carbon are near detection limits. Methane probably originates from in situ fermentation (methanogenesis) of the organic matter buried in the sediments (Claypool and Kvenvolden, 1983). In addition, the low amounts of methane together with the high organic matter contents suggest that only a small amount of organic material is converted to gas. No higher molecular weight hydrocarbons were detected.
95 mcd, both methane and organic carbon are near detection limits. Methane probably originates from in situ fermentation (methanogenesis) of the organic matter buried in the sediments (Claypool and Kvenvolden, 1983). In addition, the low amounts of methane together with the high organic matter contents suggest that only a small amount of organic material is converted to gas. No higher molecular weight hydrocarbons were detected.