GEOCHEMISTRY
Concentrations of headspace gases were routinely monitored in Hole 1240A sediments according to shipboard safety and pollution prevention considerations (Fig. F27; Table T16). Methane concentration exceeding laboratory blanks was first detected in a headspace gas sample at 19.5 mcd. Below this depth, methane increased gradually, reaching a maximum of 57 ppmv at 146.5 mcd. Methane concentrations then decreased downhole to concentrations <10 ppmv. Low ethane concentrations were detected, and no higher molecular weight hydrocarbons were observed. The reduction of dissolved sulfate in the depth range where methane increased indicates that these small amounts of methane probably originate from in situ fermentation (methanogenesis) of organic matter. The presence of interstitial sulfate inhibits methanogenesis in marine sediments (Claypool and Kvenvolden, 1983).
Interstitial Water Geochemistry
We collected 28 interstitial water samples from Hole 1240A sediments for shipboard analyses. We collected an additional 44 interstitial water samples at a frequency of one per section for the upper 60 mbsf for shore-based analyses. Chemical gradients at this site (Table T17; Fig. F28) reflect the influence of organic matter oxidation by a limited degree of sulfate reduction, the influence of fluid flow of relatively unaltered seawater in the underlying basement, the dissolution of biogenic silica driven by the relatively low thermal gradient that results from the effects of this fluid flow on conductive heat flow, and the effects of authigenic calcite precipitation. Subunit IB (see "Description of Lithologic
Unit" in "Lithostratigraphy") is relatively rich in organic carbon and is the focus of the organic carbon oxidation reactions and the resulting signatures in the interstitial water chemistry.
Chlorinity increases from 551 mM at 1.5 mcd to >560 mM by 31.1 mcd then persists at these values throughout until a decline in the deepest sample toward a value similar to seawater (Fig. F28). Salinity, measured refractively as total dissolved solids, is 35 throughout (Table T18). Sodium concentrations measured by inductively coupled plasma-atomic emission spectrophotometry averaged 1.5% lower than those estimated by charge balance reported here (Table T19). Sodium concentrations parallel chlorinity, with a total range from 472 to 489 mM.
Organic matter diagenesis, driven by microbially mediated oxidation reactions, influences the interstitial water chemistry. Sulfate decreases from 28.5 mM at 1.5 mcd to a broad minimum of <15 mM from 135.1 to 200.4 mcd, coincident with the organic carbon-rich interval of Subunit IB (see "Description of Lithologic
Unit" in "Lithostratigraphy"). Sulfate increases to 25.7 mM at 282.0 mcd. The alkalinity profile is the inverse of sulfate, with alkalinity increasing from 3.1 mM at 1.5 mcd to a maximum >17 mM from 146.5 to 189.8 mcd, within Subunit IB. Alkalinity then declines to 2.8 mM at 282.0 mcd. Alkalinity is generated by sulfate reduction and consumed partially by authigenic mineral precipitation within the sediments.
The return of sulfate and alkalinity concentrations toward seawater values near the sediment/basalt interface, along with that of other elements, indicates flow of relatively unaltered seawater in the underlying basement. Large-scale horizontal advection of such waters through oceanic crust in the central equatorial Pacific has been inferred from interstitial water geochemistry and is thought responsible for the low conductive heat flow (i.e., low thermal gradients) observed in that region (Baker et al., 1991; Oyun et al., 1995).
Dissolved manganese concentrations increase from 55 然 at 1.5 mcd to a maximum of 75 然 at 31.1 mcd, indicating the importance of suboxic diagenesis of organic matter in this depth zone. Manganese generally declines downcore, with a rapid decrease in Subunit IA to a small, secondary maximum in Subunit IB to >20 然 from 157.5 to 168.5 mcd, followed by a decline to 3 然 at 282.0 mcd. Dissolved iron is present at relatively low concentrations, presumably reflecting a limited supply of reducible iron minerals.
Phosphate concentrations increase to a maximum in Subunit IB, from 5 然 at 1.5 mcd to 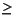 30 然 from 146.5 to 189.8 mcd, then decline to 2 然 at 282.0 mcd. Ammonium concentrations increase from below the detection limit (0.15 mM) at 1.5 mcd to a maximum >2 mM from 146.5 to 200.4 mcd then decline to 0.17 mM (just above the detection limit) at 282.0 mcd. The increases in phosphate and ammonium result from the oxidation of organic matter, whereas the decreases with depth below Subunit IB indicate the influence of seawater flow in the underlying oceanic crust.
30 然 from 146.5 to 189.8 mcd, then decline to 2 然 at 282.0 mcd. Ammonium concentrations increase from below the detection limit (0.15 mM) at 1.5 mcd to a maximum >2 mM from 146.5 to 200.4 mcd then decline to 0.17 mM (just above the detection limit) at 282.0 mcd. The increases in phosphate and ammonium result from the oxidation of organic matter, whereas the decreases with depth below Subunit IB indicate the influence of seawater flow in the underlying oceanic crust.
Dissolved silicate increases from 601 然 at 1.5 mcd to ~1000 然 by 273.0 mcd then declines in the deepest sample. Site 1240 has a significantly lower thermal gradient (~2.56蚓/100 m) than Site 1238 (~12.7蚓/100 m) or Site 1239 (~8.8蚓/100 m). The silicate increase with depth for Site 1240 is also much less steep than those observed at Sites 1238 and 1239, and Site 1240 never reaches temperatures high enough to produce silicate concentrations >1800 然 as seen at depth in Sites 1238 and 1239.
Barium concentrations are low (<7 然) throughout, indicating that sulfate concentrations are sufficient to prevent significant dissolution of barite. Boron concentrations are close to seawater values in the shallowest and deepest samples (436 然 at 1.5 mcd and 433 然 at 282.0 mcd, respectively), with a middepth maximum to 600 然 or greater.
Calcium decreases from 10.3 mM at 1.5 mcd to a minimum of <3.5 mM from 103.5 to 178.9 mcd. This calcium minimum is present at a shallower depth than the alkalinity maximum. Calcium increases to 9.5 mM at 282.0 mcd. The shallow part of this profile is controlled by authigenic calcite precipitation driven by the alkalinity increase, and the deeper portion reflects the influence of seawater advection in the underlying oceanic crust. Magnesium concentrations average ~54 mM, with little variation with depth. Magnesium/calcium ratios increase from 5.1 at 1.5 mcd to >17 from 125.3 to 189.8 mcd then decrease to 5.7 at 282.0 mcd (Fig. F28). The increase in magnesium/calcium in the shallower sediments, driven by the decrease in calcium, indicates that calcite precipitation is the dominant authigenic mineralization reaction, consuming calcium and alkalinity. Despite the resulting high magnesium/calcium ratios, the formation of dolomite is apparently inhibited by the presence of sulfate in interstitial water throughout Site 1240.
Lithium concentrations show little variation with depth, with the exception of a small maximum in Subunit IC to 33 然 at 229.8 mcd. Strontium concentrations are close to seawater values in the shallowest and deepest samples and increase in Subunit IB from 91 然 at 146.5 mcd to a maximum in Subunit IC of 177 然 at 229.8 mcd. Potassium concentrations have a small initial increase then have a small overall decline with depth.
Sedimentary Inorganic Carbon, Organic Carbon, Nitrogen, and Sulfur Concentrations
Inorganic carbon (IC), total carbon (TC), total nitrogen (TN), and total sulfur (TS) concentrations were determined on sediment samples from Hole 1240A (Table T18). Organic matter carbon/nitrogen ratios and Rock-Eval pyrolysis were employed to characterize the organic matter.
Calcium carbonate concentrations range between 22.8 and 80.3 wt% (average = 56.0 wt%) (Table T18; Fig. F29). Carbonate concentrations decrease from ~60 to ~40 wt% in the uppermost 10.1 m and increase to a maximum of 80.3 wt% between 10.1 and 44.1 mcd. Calcium carbonate concentrations reach a minimum from 137.4 to 206.2 mcd, with values typically <50 wt% and as low as 22.8 wt% at 175.2 mcd. Below 206.2 mcd, carbonate contents increase abruptly to >35 wt% with large-amplitude variations at depth.
TOC concentrations range between 0.2 and 3.1 wt% (average = 1.0 wt%) (Table T18; Fig. F29). In the uppermost 140 m, TOC concentrations range between 0.3 and 1.5 wt%, with the highest concentrations in the upper ~35 m. TOC concentrations are >1 wt% and up to 3.1 wt% (average = 2.0 wt%) from 142.7 to 212.3 mcd, with the TOC maximum coincident with the calcium carbonate minimum. At greater depths, TOC contents are generally <1 wt%. Variations in TOC are apparently not driven solely by dilution by calcium carbonate, as variations in TOC on a carbonate-free basis (CFB) are similar to those in TOC (Fig. F29). Higher TOC concentrations are associated with lower calcium carbonate concentrations (Fig. F30).
Variations in TN concentration are similar to those in TOC (Fig. F29). In the uppermost 142.7 mcd, TOC/TN ratios average 4.8, typical of unaltered marine organic matter (Bordovskiy, 1965; Emerson and Hedges, 1988; Meyers, 1997). In the TOC-rich interval from 142.7 to 212.3 mcd, TOC/TN ratios range from ~2 to 9. Below 212.3 mcd, where both TOC and TN contents are low, TOC/TN ratios average 9.0. The interval of high TOC concentrations is unlikely to be the result of enhanced terrigenous organic matter input because high TOC concentrations are associated with lower TOC/TN ratios. The increase in TOC/TN ratios at depths >212.3 mcd may be the result of preferential loss of nitrogen relative to carbon during organic matter diagenesis (Meyers, 1997).
Nine samples were selected for Rock-Eval pyrolysis from the depth interval of the TOC maximum. Low Tmax values indicate that the organic matter is thermally immature (Table T19). The relationship between S2 and TOC concentrations indicates Type II organic matter (Fig. F31) (i.e., marine algal organic matter) (Tissot and Welte, 1984; Langford and Blanc-Valleron, 1990). This is consistent with the interpretation that the supply of terrigenous organic matter has played only a minor role at this site.
Fresh marine plankton has a relatively high lipid content, thus high H/C ratios. Therefore, well-preserved organic matter of marine algal origin (Type II) yields high hydrogen index (HI) values when subjected to pyrolysis. The HI values vary between 254 and 379 (average = 352) (Table T19; Fig. F31). These values are typical for high-productivity areas (e.g., upwelling) associated with a high organic carbon flux and rapid burial (Whelan et al., 1990). High sedimentation rates mark the interval of high TOC concentrations (~143-212 mcd) and may have favored the preservation of the organic material (see "Biostratigraphy" and "Age Model and Mass Accumulation Rates"). The presence of diatom oozes during this Pliocene-Pleistocene interval supports a hypothesis of enhanced productivity for this interval (see "Lithostratigraphy," and "Diatoms" in
"Biostratigraphy").

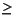 30 然 from 146.5 to 189.8 mcd, then decline to 2 然 at 282.0 mcd. Ammonium concentrations increase from below the detection limit (0.15 mM) at 1.5 mcd to a maximum >2 mM from 146.5 to 200.4 mcd then decline to 0.17 mM (just above the detection limit) at 282.0 mcd. The increases in phosphate and ammonium result from the oxidation of organic matter, whereas the decreases with depth below Subunit IB indicate the influence of seawater flow in the underlying oceanic crust.
30 然 from 146.5 to 189.8 mcd, then decline to 2 然 at 282.0 mcd. Ammonium concentrations increase from below the detection limit (0.15 mM) at 1.5 mcd to a maximum >2 mM from 146.5 to 200.4 mcd then decline to 0.17 mM (just above the detection limit) at 282.0 mcd. The increases in phosphate and ammonium result from the oxidation of organic matter, whereas the decreases with depth below Subunit IB indicate the influence of seawater flow in the underlying oceanic crust.