-
CH4 + SO42-
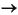 HCO3- + HS- + H2O
HCO3- + HS- + H2O
A total of 113 whole-round samples were collected for interstitial water (IW) analyses at this site (12 samples from Hole 1244B, 72 samplesT7NOTE from Hole 1244C, 23 samples from Hole 1244E, and 6 samples from Hole 1244F). Routine samples were collected at a frequency of approximately two whole-round samples per core in the upper 150 m, followed by a sampling resolution of one whole-round sample per core below this depth. A higher-resolution sampling protocol was used within the anaerobic methane oxidation (AMO) zone in Hole 1244C (approximately two whole-round samples per section) in a coordinated program with the shipboard microbiologists. The purpose of this high-resolution sampling was threefold: (1) to clearly define biogeochemical zones within the sedimentary sequence, (2) to estimate the presence and abundance of gas hydrate within the GHSZ using interstitial chloride concentration as proxy for the presence of gas hydrate, and (3) to provide supporting data for studies on the microbial dynamics of the sulfate/methane interface (SMI). The IW geochemistry data are tabulated in Table T4 and are illustrated in Figure F14.
The downhole chloride content exhibits overall features similar to those previously observed in IW samples recovered from gas hydrate-bearing sediments during ODP drilling (e.g., Cascadia margin: Westbrook, Carson, Musgrave, et al., 1994; Blake Ridge: Paull, Matsumoto, Wallace, et al., 1996; Chile Triple Junction: Behrmann, Lewis, Musgrave, et al., 1992). The most striking feature of the chloride profile at Site 1244 is the zone defined by excursions to significantly lower values (519 mM) in the GHSZ, followed by a linear decrease from 70 mbsf to the bottom of the hole, where the chloride concentration is 472 mM (Fig. F15).
The linear profile of dissolved Cl- is strongly suggestive of diffusion between low-salinity fluids in the accretionary wedge and a chloride concentration of 540 mM at 70 mbsf, apparently with a negligible advective component. The onset of low-chloride fluids corresponds to the depth of seismic Reflector AC, which is thought to represent the top of the accreted sedimentary wedge (see "Introduction"). The high lithium content measured in the deeper samples recovered from this site is consistent with a source deeper than 1 km, which is similar to the interpretation based on IW data from Sites 889 and 890 (Fig. F16) on the Cascadia margin off Vancouver Island (Kastner et al., 1995). Inferences about the source depth of low-chlorinity, high-lithium fluids are supported by field and laboratory observations that document remobilization of lithium from aluminosilicates at temperatures ranging from 70░ to 100░C (Edmond et al., 1979; Seyfried et al., 1984). The geothermal gradient for this site is 61░C/km (see "Downhole Tools and Pressure Coring").
Superimposed on the IW freshening trend that results from contact with the deeper fluids, excursions with anomalous low-chlorinity values above the BSR are thought to represent gas hydrate dissociation during core retrieval and can be used to estimate the amount of hydrate in the sediments (Hesse and Harrison, 1981; Kastner et al., 1995; Behrmann, Lewis, Musgrave, et al., 1992; Paull, Matsumoto, Wallace, et al., 1996; Hesse, 2003). The uncertainties in these estimates arise from a paucity of information on (1) the in situ chlorinity values of the IW, (2) the chloride content potentially trapped within the pores of the gas hydrates, (3) the spatial sampling bias, and (4) fluid transport and diffusion rates.
Because we have no data on the amount of Cl- sequestered by the hydrate cage, we have assumed here (as has been previously done) that hydrate formation excludes all dissolved ions and that dissociation of hydrate will release water with a chloride concentration of 0 mM.
Gas hydrate is present at irregular intervals within the GHSZ, as evidenced by the recovery of gas hydrate samples and thermal anomalies seen in IR data (see "Infrared Scanner" in "Physical Properties"). Because of this irregular gas hydrate distribution, uncertainties are introduced into gas hydrate distribution estimates based on chloride concentration data. For example, in Core 204-1244C-7H, the IR data indicate the presence of hydrate at 57-58 mbsf (prior to cutting the core) but no hydrate was actually sampled from this core. The IW sample at 54.8 mbsf does indeed show an excursion to 514 mM, whereas the sample collected at 64.37 mbsf shows no significant anomaly (Cl- = 556 mM). Hydrate was sampled in Section 204-1244C-8H-2, but an IW sample that was collected ~5 m away from it shows no chloride anomaly. Another sample of nodular hydrate was recovered in Section 204-1244C-10H-2; in this case, the IW sample collected from sediments adjacent to the gas hydrate has a chloride concentration of 533 mM. Similarly, hole-to-hole variability in the predicted presence of gas hydrate based on the chloride data reflects lateral heterogeneity in hydrate distribution as well as limitations of sampling resolution, which, at two samples per core, is not enough to identify every occurrence of gas hydrate. For example, a chloride anomaly recorded at ~70 mbsf in Hole 1244E was not observed in Hole 1244C (Fig. F15).
The lower sampling resolution in Hole 1244E does not allow identification of the majority of gas hydrate horizons observed in Hole 1244C. In addition to the whole-round samples collected on the catwalk, we collected three IW samples from the working half of Core 204-1244E-8Y. This core was collected with the FPC, and the density logs were obtained under pressure to image the presence of gas hydrate (see "HYACINTH Pressure Coring and Logging" in "Downhole Tools and Pressure Coring"). The chloride analyses, listed in Table T5, show a well-defined anomaly in Sample 204-1244E-8Y-1, 10-15 cm, which was collected from the zone where density anomalies indicate the presence of gas hydrate.
It is difficult to determine the background in situ Cl- content, particularly in the upper 70 m of the sedimentary sequence. Chloride concentration from 70 mbsf to the BSR form a linear trend that extends below 250 mbsf, and we have used this trend as a baseline for in situ Cl- concentration, as shown in Figure F17. However, in the upper 15 m of Site 1244, there is an increase in dissolved Cl- equivalent to 0.84 mM/m. A similar increase in dissolved Cl- was reported at hydrate-bearing sites from the Blake Ridge (Paull, Matsumoto, Wallace, et al., 1996) and the Cascadia margin (Kastner et al., 1995). Two possible scenarios could account for this increase. It might represent a diffusion gradient resulting from ion exclusion during gas hydrate formation. Alternatively, the positive gradient in the shallow samples might reflect nonsteady-state conditions induced by glacial-interglacial salinity variations driven by changes in global ice volume (McDuff, 1985; Schrag and DePaolo, 1993).
Because of uncertainties in our knowledge of the background dissolved Cl- in the upper 80 mbsf at Site 1244, we have estimated a range of values for gas hydrate occupying sediment pore space at Site 1244 (Fig. F17). These data suggest that in the GHSZ, 2%-8% of the pore space is occupied by gas hydrate. With the present data set it is not possible to ascertain the exact value and distribution of hydrate occupancy; however, several lines of evidence lead us to believe that the amount of hydrate is closer to our conservative estimate (Fig. F17B). The hydrate onset, which is predicted to occur at 40 mbsf using the minimum hydrate estimates, corresponds to the base of a zone where there are no major excursions in the chloride distribution. The upper 40 m also shows a more coherent pattern in downhole LWD resistivity data (see "Downhole Logging"). In contrast, LWD resistivity data between 40 mbsf and the depth of the BSR exhibit high variability, which perhaps reflects the patchiness of the gas hydrate distribution. These data all suggest that in the upper 40 mbsf there is not enough methane within the sediments to stabilize a gas hydrate structure (see "Hydrocarbon Gases" in "Organic Geochemistry"). Although the two end-member interpretations affect the predicted stratigraphic distribution of gas hydrate, the total gas hydrate inventory predicted by each interpretation differs by only ▒10%.
In addition, we have compared the chloride profile from Site 1244 with that of Site 888, which was drilled west of the Cascadia accretionary margin as a reference site during Leg 146 (Westbrook, Carson, Musgrave, et al., 1994). The sedimentary section at Site 888 is unaffected by the accretionary tectonics that deform and consolidate sediments within the wedge. At this site there is no seismic, geochemical, or physical evidence for the presence of gas hydrate (Westbrook, Carson, Musgrave, et al., 1994). A comparison of the chloride distribution between Sites 888 and 1244 (Fig. F18) suggests that the true background in situ Cl- content in Site 1244 is closer to that assumed for our conservative gas hydrate estimate (Fig. F17B). Because there is no evidence for hydrate formation at Site 888, we conclude that the increase in Cl- in the upper 80 mbsf is likely to reflect global ocean changes rather than hydrate formation. Moreover, we also conclude that the presence of gas hydrate at Site 1244 is limited to between 40 and 130 mbsf.
The SMI is a fundamental microbial and geochemical boundary in marine sediments and was the subject of integrated microbiological and geochemical investigations during Leg 204. Above the interface, sulfate reducers utilize interstitial sulfate to oxidize sedimentary organic matter; below the interface, methanogens generate methane. At the interface, a unique consortium of microbes interacts in AMO, a net biogeochemical process described by
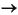 HCO3- + HS- + H2O
HCO3- + HS- + H2O
(Reeburgh, 1976) that involves carbon and sulfur cycling at the interface and also affects the geochemisty above and below the zone of AMO (Alperin et al., 1988; Borowski et al., 1997; Hoehler et al., 2000; Rodriguez et al., 2000).
In Hole 1244C, the SMI is located at ~8.5 mbsf, where interstitial sulfate first reaches a minimum concentration concomitant with increasing methane concentration as documented by headspace gas data (see "Organic Geochemistry") (Fig. F19). The zone where AMO occurs was sampled extensively by the microbiology team at this site (see "Microbiology").
The maximum sulfate concentration is 29.7 mM just below the sediment/water interface (Sample 204-1244C-1H-1, 65-75 cm) and falls below 1 mM at 9.15 mbsf (Sample 2H-3, 66-75 cm) (Table T4; Fig. F19). Below this depth, sulfate does not reach zero but remains at concentration <1 mM. These nonzero sulfate concentrations are likely to represent sulfate contamination from seawater in the borehole. Larger amounts of sulfate (>1 mM), derived from seawater circulating in the borehole, invaded the IW samples during XCB drilling below 189 mbsf. The sulfate profile is approximately linear between 4 and 7.5 mbsf, with curvature at both the top and the bottom of the profile, which probably represents sulfate depletion from oxidation of sedimentary organic matter (SOM) and transport by advecting fluids.
Borowski et al. (1996) hypothesized that downward sulfate flux is balanced by upward methane flux under conditions of high upward methane diffusion (such as is present in gas hydrate terranes) and low sulfate reduction rates in the sulfate reduction zone. These conditions are most often present within continental margin sediments that harbor gas hydrate. Under these conditions, sulfate and methane are coupled geochemical species because AMO occurring at the SMI involves the microbially mediated co-consumption of sulfate and methane. Because the stoichiometric ratio between sulfate and methane consumed by AMO is 1/1, these respective fluxes should be equivalent at the interface. Thus, the rate of upward methane diffusion can be estimated using the sulfate gradient, assuming that the middle and lower portions of the sulfate profile are dominated by sulfate depletion resulting from AMO. Sulfate gradients are an important proxy for methane flux because true methane gradients have only rarely been measured as a result of methane gas loss that occurs during core retrieval (Dickens et al., 1997; Paull and Ussler, 2001).
Fick's First Law allows the delivery of sulfate to the SMI to be calculated. If the diffusion coefficient of sulfate (Do), sediment porosity (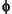 ), and the sulfate concentration gradient (
), and the sulfate concentration gradient ( C) with depth (
C) with depth ( x) are known, the diffusive flux (J) may be estimated by
x) are known, the diffusive flux (J) may be estimated by
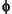 3
3  C/
C/ x.
x.
Compared to other localities displaying linear sulfate profiles (e.g., the Blake Ridge and ODP Sites 994, 995, and 997) (Paull, Matsumoto, Wallace, et al., 1996), the sulfate distribution at Site 1244 in the lower sulfate reduction zone may not be sufficiently dominated by AMO to produce a meaningful estimate of methane flux. Nevertheless, an estimated methane flux at Site 1244 of 2.7 x 10-3 mM/cm2/yr was calculated based on a sulfate gradient of 5.5 mM/m between 4 and 8 mbsf (equivalent to a slope of 0.18 m/mM) (see Fig. F19B), a sulfate diffusion coefficient of 5.8 x 10-6 cm2/s at 5░C and average porosity of 0.65. For comparison, at the Blake Ridge (offshore southeastern North America), the steepest measured sulfate gradient predicts a methane flux of 1.8 x 10-3 mM/cm2/yr (Borowski et al., 1996, 2000), so the flux at Site 1244 is ~33% higher than that reported to date for any large passive margin gas hydrate terrane. These estimates assume methane delivery through diffusion only and assume that the linear portion of the sulfate curve is mainly created by sulfate demand at the SMI. If significant water or methane advection occurs or if sulfate depletion through AMO is of minor importance, then this estimate is invalid.
The early diagenesis of marine sediments is most often dominated by organic matter decomposition (e.g., Berner, 1980). Interstitial alkalinity, ammonium (NH4+), and phosphate (PO43-) concentration increase rapidly with increasing depth, reaching maximum concentration at 58.97, 64.37, and 17.90 mbsf, respectively (Table T4; Fig. F14). These IW constituents reflect the activity of interstitial microbes that are metabolizing SOM through a reaction cascade that ultimately produces inorganic metabolic by-products. From the shape of the profiles, it is likely that the microbial decomposition of SOM is most active in the upper 100 m of the sedimentary section, where production outpaces depletion for each constituent. During postcruise research, we will have the opportunity to correlate microbial abundance and activity to key interstitial constituents that identify organic matter decomposition.
As discussed in the previous sections, the distribution of dissolved chloride and sulfate provide the major clues to understanding the processes involved in gas hydrate dynamics and methane fluxes at this site. Further clues about the nature of fluid sources, diagenetic reactions, and microbiological processes within these sediments can be obtained from the distribution of other dissolved ions in the pore fluids shown in Figure F14 and listed in Table T4.
We have documented a zone of low chloride and high lithium concentration within the accreted sediments of the wedge (Fig. F16), which is similar in composition to that reported for Sites 889 and 890 in the northern section of the accretionary prism (Kastner et al., 1995). Superimposed on the lithium increase there is a significant decrease in the concentration of this element in pore fluids recovered from 160 to 180 mbsf (Fig. F14). This depth interval corresponds to the reflector identified as Horizon B on the seismic data (see "Introduction"). The lithium distribution within this depth interval reflects incorporation of this element into solid geochemical phases associated with low-temperature diagenetic alteration of material present in Horizon B, even though we have not identified the phases that are incorporating lithium at this time. The sediments from this depth horizon also show variations in their physical properties and are characterized by high-density (~1.9 g/cm3) and low porosity (~50%) values (see "Physical Properties"). Postcruise analyses of lithium and its isotopic composition in various geochemical phases will provide constraints on the diagenetic processes occurring at Horizon B.
In addition to low chlorinity and high lithium values, the deep-seated fluid at Site 1244 is also enriched in Sr2+ (Fig. F14). The dissolved strontium signature within the GHSZ at this site (0-130 mbsf) is more complex than that of lithium because strontium is involved in carbonate diagenetic reactions, which in turn are dependent on temperature, pressure, and pH. The increase in dissolved boron within the gas hydrate-bearing section may also be related to carbonate diagenesis (e.g., Deyhle et al., 2001), as evidenced by the similarity between alkalinity and boron distributions.
Iron and manganese have similar profiles, showing coincident maxima in their concentration (Fe = 52 ÁM and Mn = 6 ÁM) at depths ranging from 29 to 31 mbsf or ~20 m below the depth of sulfate depletion (Fig. F14; Table T4). This distribution may reflect cycling of iron manganese minerals, which precipitate as sulfides below the SMI and are remobilized at a depth in the sediment section where sulfide is depleted. A similar distribution was observed at Site 1230 on the Peru margin (D'Hondt, J°rgensen, Miller, et al., 2003). The dissolved manganese concentration remains level at ~1 ÁM within the GHSZ, followed by an increase downhole, which is probably associated with the composition of the deep-seated fluids. In contrast, the dissolved iron remains higher than background (<1 ÁM) throughout the GHSZ. Postcruise analyses of the distribution and isotopic composition of dissolved sulfide and of solid sulfide minerals will likely provide constraints to the nature of the Fe-Mn biogeochemical cycling at this site.
The distribution of dissolved and solid-phase barium in continental margin sediments has received recent attention because preservation of barite fronts within the sediments may serve as an indication of methane fluxes in the past. Dickens (2001) has presented a model based on the diagenetic behavior of barium-bearing phases in marine reducing sediments. Barite is a widespread component of oceanic sediment and is particularly abundant in areas characterized by high-productivity waters (e.g., Dehairs et al., 1980; Collier and Edmond, 1984; Dymond et al., 1992; Paytan and Kastner, 1996). In spite of the low solubility and, hence, high stability of barite in seawater, this mineral is dissolved under conditions of sulfate depletion:
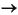 Ba2+ + SO42-.
Ba2+ + SO42-.
Such conditions are frequently present in organic-rich coastal sediments accumulating at high rates, in which sulfate concentration in the IWs approaches zero, as a result of microbial sulfate reduction of SOM (Brumsack and Gieskes, 1993). In these settings, dissolved barium accumulates in the pore fluids, reaching concentrations that are several orders of magnitude higher than those observed in seawater (e.g., Torres et al., 1996). The dissolved barium distribution at Site 1244 follows this general trend, reaching a concentration of ~60 ÁM at 20 mbsf (Fig. F20).