INORGANIC GEOCHEMISTRY
Interstitial Water Chemistry
Thirteen interstitial water samples were collected at Site 1212. In Hole 1212A, nine samples were taken between 0 and 90 mbsf (one sample/core). Hole 1212B was sampled only when depths exceeded those reached in Hole 1212A. The sampling resolution was decreased (one sample per three cores) in Hole 1212B such that four samples were collected between 100 and 200 mbsf. Details of analytical methods can be found in "Inorganic Geochemistry" in the "Explanatory Notes" chapter. Filtered (0.45 Ám) samples were analyzed for pH, salinity, chlorinity, alkalinity, sulfate (SO42-), phosphate (HPO42-), ammonium (NH4+), silica (Si(OH)4), boron (H3BO3), iron (Fe2+), manganese (Mn2+), and major cations (Na+, K+, Mg2+, Ca2+, Li+, Sr2+, and Ba2+). A compilation of data is provided in Table T11. Cited values for average seawater composition are from Millero and Sohn (1992) and Broecker and Peng (1982).
pH, Salinity, Chloride, and Sodium
The pH of pore waters at Site 1212 ranges between 7.37 and 7.63, with an average value of 7.45 ▒ 0.07 (Table T11). All values are lower than the average seawater value of 8.1. As at Sites 1209-1211, variability in the pH profile occurs within the upper lithologic unit, which is characterized by a lesser proportion of carbonate sediment (72 wt% carbonate through Subunit IA) relative to underlying sediments (82 wt% carbonate in Subunit IB) (see "Carbonate" in "Organic Geochemistry"). Below ~40 mbsf, the pH becomes relatively uniform as the carbonate content of the sediments increases, reflecting the buffering capacity of carbonate-dominated sediments. Salinity ranges from 34.0 to 35.5 g/kg.
The chloride (Cl-) pore water profile fluctuates around a mean of 559 ▒ 2 mM, with an increase in pore water Cl- anion content between ~30 and 90 mbsf (Fig. F21). Sodium (Na+) concentrations, calculated using the methods described in Broecker and Peng (1982), average 474 ▒ 3 mM (Fig. F22). Such broad downcore variations in pore water Na+ and Cl- profiles of pelagic sediments have been linked to variations in mean ocean salinity associated with changes in ice volume (McDuff, 1985; Schrag et al., 1995).
Alkalinity, Sulfate, Ammonium, Phosphate, Iron, and Manganese
The concentrations of SO42-, alkalinity, NH4+, and HPO42- in the interstitial waters at Site 1212 (Fig. F22) are relatively low and invariant. Downcore, SO42- concentrations decrease steadily from 28 mM (average seawater concentration) in the uppermost sample to 22 mM at 88.35 mbsf. Below this depth, SO42- remains uniform at 22 mM down to the bottom of the profile (186.60 mbsf). Alkalinity decreases from 2.8 mM in the surface pore waters (10.85 mbsf) to 2.2 mM at 159.10 mbsf. Here, the trend reverses, and the alkalinity increases to 2.8 mM at 186.60 mbsf.
Similar to Sites 1209-1211, the NH4+ concentrations are low (between 5 and 130 ÁM), implying that the organic matter content of the sediment at Site 1212 is low (see "Carbonate" in "Organic Geochemistry"). This interpretation is supported by the limited downcore decrease in SO42- and the low pore water HPO42- concentrations, the highest values of which are <2 ÁM (1.5-1.8 ÁM) (Fig. F22). However, Site 1212 has the greatest degree of sulfate reduction on the Southern High. A corresponding increase in pyrite was observed through lithologic Unit I in cores from Site 1212 relative to other Southern High sites (see "Lithologic Unit
I" in "Lithostratigraphy").
The Mn2+ profile exhibits a downcore decrease from 16 ÁM at 2.95 mbsf to 1 ÁM at 39.35 mbsf. Below this depth, there is little variation in the Mn2+ profile and concentrations remain low (1-4 ÁM). As at Sites 1209 and 1210, elevated concentrations of Mn2+ in the upper part of the sediment section are likely related to the occurrence of a condensed interval containing Mn-rich phases. Consequently, the minor excursion at ~60 mbsf is interpreted to reflect the dissolution of Mn minerals and diffusion of Mn2+ away from Mn-rich sediments.
After an increase to 4 ÁM in the uppermost sediments, the downcore Fe2+ concentrations decrease significantly through the upper ~50 mbsf of the section. Concentrations decrease from 42 ÁM at 2.95 mbsf to 4 ÁM at 53.35 mbsf; the lower part of the profile is uniform with average concentrations of 3 ▒ 1 ÁM (Fig. F23). The inflection at ~50 mbsf coincides with the middle Miocene to lower middle Eocene unconformity in Core 198-1212A-7H (see "Lithostratigraphy"). The elevated concentrations of Fe2+ through the sediment section corresponding to lithologic Unit I (0.00-53.60 mbsf) are likely related to the close proximity of volcanic ash (Subunit IA) and other Fe2+ sources to sites of pyrite formation.
Potassium, Calcium, Magnesium, Strontium, and Lithium
The concentrations of K+, Ca2+, and Mg2+ in the upper pore waters at Site 1212 differ little from concentrations in average seawater (12, 11, and 51 mM, respectively) (Fig. F24). In lithologic Unit II (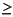 ~60 mbsf) of the section, downcore trends are consistent with those resulting from exchange with basaltic basement at depth (Gieskes, 1981), wherein K+ and Mg2+ concentrations decrease downcore and Ca2+ increases. However, in lithologic Unit I, it is likely that processes other than exchange with basement are affecting the distribution of Mg2+ cations. In the pore waters contained within Unit I (see "Lithologic Unit
I" in "Lithostratigraphy"), Mg2+ concentrations decrease at a rate that is inconsistent with what might be expected for a diffusional gradient associated with basement exchange. Consequently, it is likely that a Mg-rich phase is precipitating within lithologic Unit I. Below ~50 mbsf, where the lithology is dominated by carbonate-rich phases, Mg2+ concentrations become uniform (46.7 ▒ 0.3 mM). Assuming that volcanic weathering reactions with basement are removing Mg2+ from interstitial waters at depth, the trend observed below ~50 mbsf implies that Mg2+ cations are being mobilized into the pore waters. Although there is a 5-mM increase in Ca2+ through this interval, the Sr profile and Sr/Ca ratios imply that carbonate dissolution is not responsible for the increase in Mg2+.
~60 mbsf) of the section, downcore trends are consistent with those resulting from exchange with basaltic basement at depth (Gieskes, 1981), wherein K+ and Mg2+ concentrations decrease downcore and Ca2+ increases. However, in lithologic Unit I, it is likely that processes other than exchange with basement are affecting the distribution of Mg2+ cations. In the pore waters contained within Unit I (see "Lithologic Unit
I" in "Lithostratigraphy"), Mg2+ concentrations decrease at a rate that is inconsistent with what might be expected for a diffusional gradient associated with basement exchange. Consequently, it is likely that a Mg-rich phase is precipitating within lithologic Unit I. Below ~50 mbsf, where the lithology is dominated by carbonate-rich phases, Mg2+ concentrations become uniform (46.7 ▒ 0.3 mM). Assuming that volcanic weathering reactions with basement are removing Mg2+ from interstitial waters at depth, the trend observed below ~50 mbsf implies that Mg2+ cations are being mobilized into the pore waters. Although there is a 5-mM increase in Ca2+ through this interval, the Sr profile and Sr/Ca ratios imply that carbonate dissolution is not responsible for the increase in Mg2+.
The Sr2+ and Sr/Ca pore water profiles both increase steadily from the shallowest sample at 2.95 mbsf down to 29.85 mbsf, suggesting that carbonate dissolution or recrystallization is occurring within this interval (Fig. F24) (e.g., Baker et al., 1982). Pore waters collected within lithologic Subunit IB and lithologic Unit II show little change in either the Sr2+ or Sr/Ca profiles, implying that there is little additional Sr2+ input from carbonate alteration.
The Li+ concentrations decrease sharply from 30 ÁM in the shallowest sample (2.95 mbsf) to a minimum of 16 ÁM at 10.85 mbsf (Fig. F24). Below this depth, concentrations gradually increase to 22 ÁM at the base of the profile (186.60 mbsf). The decrease to Li+ concentrations below that of average seawater (25 ÁM) may reflect uptake by clay minerals forming through the weathering of volcanic material (Gieskes, 1981).
Silica
The shape of the dissolved silica profile at Site 1212 is generally similar to those observed at Sites 1207-1211 (Fig. F25). Pore water silica concentrations are highest in lithologic Unit I, ranging between 550 and 660 ÁM. Through the underlying sediment section, pore water Si(OH)4 concentrations decrease gradually to 144 ÁM at the base of the profile (159.10 mbsf). Elevated concentrations in the upper part of the profile are interpreted to reflect the leaching and weathering of volcanic ash and biogenic silica in the Pliocene-Pleistocene sediments. Lower concentrations coincide with the appearance of carbonate-dominated sediments and chert in lithologic Units II and III. The removal of Si(OH)4 from pore waters may be the result of the recrystallization of opal-A to opal-CT or quartz (Baker, 1986; Gieskes, 1981).
Boron and Barium
Given that variations in the Ba2+ and H3BO3 concentrations in pore waters of pelagic sediments are poorly understood, profiles for these parameters at Site 1212 are described largely for purposes of documentation (Table T11). The average boron concentration (378 ▒ 21 ÁM) is higher than that of average seawater (416 ÁM). The Ba2+ concentration averages 0.3 ▒ 0.1 ÁM, and the profiles show little variability with depth. Concentrations are extremely low, but on average, are higher than those of average seawater, implying that Ba2+ is being added to the system. Possible sources of Ba2+ include calcareous skeletal debris and minor volcanic ash in the Neogene section, which may be undergoing leaching and/or dissolution.

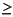 ~60 mbsf) of the section, downcore trends are consistent with those resulting from exchange with basaltic basement at depth (Gieskes, 1981), wherein K+ and Mg2+ concentrations decrease downcore and Ca2+ increases. However, in lithologic Unit I, it is likely that processes other than exchange with basement are affecting the distribution of Mg2+ cations. In the pore waters contained within Unit I (see "Lithologic Unit
I" in "Lithostratigraphy"), Mg2+ concentrations decrease at a rate that is inconsistent with what might be expected for a diffusional gradient associated with basement exchange. Consequently, it is likely that a Mg-rich phase is precipitating within lithologic Unit I. Below ~50 mbsf, where the lithology is dominated by carbonate-rich phases, Mg2+ concentrations become uniform (46.7 ▒ 0.3 mM). Assuming that volcanic weathering reactions with basement are removing Mg2+ from interstitial waters at depth, the trend observed below ~50 mbsf implies that Mg2+ cations are being mobilized into the pore waters. Although there is a 5-mM increase in Ca2+ through this interval, the Sr profile and Sr/Ca ratios imply that carbonate dissolution is not responsible for the increase in Mg2+.
~60 mbsf) of the section, downcore trends are consistent with those resulting from exchange with basaltic basement at depth (Gieskes, 1981), wherein K+ and Mg2+ concentrations decrease downcore and Ca2+ increases. However, in lithologic Unit I, it is likely that processes other than exchange with basement are affecting the distribution of Mg2+ cations. In the pore waters contained within Unit I (see "Lithologic Unit
I" in "Lithostratigraphy"), Mg2+ concentrations decrease at a rate that is inconsistent with what might be expected for a diffusional gradient associated with basement exchange. Consequently, it is likely that a Mg-rich phase is precipitating within lithologic Unit I. Below ~50 mbsf, where the lithology is dominated by carbonate-rich phases, Mg2+ concentrations become uniform (46.7 ▒ 0.3 mM). Assuming that volcanic weathering reactions with basement are removing Mg2+ from interstitial waters at depth, the trend observed below ~50 mbsf implies that Mg2+ cations are being mobilized into the pore waters. Although there is a 5-mM increase in Ca2+ through this interval, the Sr profile and Sr/Ca ratios imply that carbonate dissolution is not responsible for the increase in Mg2+.