 of the average previously observed trend over the depths between 8.9 mbsf (Sample 201-1226B-2H-3, 145-150 cm) and 419.4 mbsf (Sample 47X-2, 145-150 cm).
of the average previously observed trend over the depths between 8.9 mbsf (Sample 201-1226B-2H-3, 145-150 cm) and 419.4 mbsf (Sample 47X-2, 145-150 cm).
Microbiological samples were taken from every core of Hole 1226B at intervals of ~9.5 m for complete profiles of sulfate reduction rates, carbon, hydrogen, nitrogen, and sulfur (CHNS) geochemistry and deoxyribonucleic acid (DNA) analysis (Fig. F5). This basic sampling scheme was maintained throughout Hole 1226B. More extensive sampling focused on variable depth intervals in Hole 1226B throughout the sediment column but particularly emphasized the top and bottom layers of the sediment column, which are characterized by the steepest geochemical gradients (see Fig. F4). Between the sediment surface and Core 201-1226B-12H, the third section of every second core was sampled in an extensive manner. In the central portion of the sediment column (Cores 201-1226B-12H through 30X), the third section of every third core was taken. Additionally, samples for fluorescence in situ hybridization (FISH), adenosine triphosphate (ATP), and lipid biomarker analysis were fixed or frozen, as appropriate, for selection and processing of the most promising and representative material on shore. Also, whole-round cores were subsampled into 5-cm3 syringes for subsequent measurement of methanogenesis, acetate turnover, and thymidine incorporation into DNA. Samples for acridine orange direct count (AODC) were fixed from the same intervals, and the cells were counted on the ship. Samples for anaerobic oxidation of methane, pyrolysis of organic material, and diffusion experiments were taken at several defined intervals (Fig. F5).
Based on our initial experience at Site 1225 (see "Contamination Tests" in "Microbiology" in the "Site 1225" chapter), modifications to minimize contamination and to increase handling efficiency were introduced at Site 1226. Details are given in "Microbiology" in the "Explanatory Notes" chapter. In contrast to Site 1225, interstitial water samples were always taken on the catwalk, either from intervals adjacent to the microbiology samples or from additional core sections.
Samples were taken for microbial enrichments and MPN quantifications with the aim of comparing distinct depths and chemical zones in the sediment column. Samples for slurry preparation and for cultivation of manganese reducers were taken from the surface or near-surface sediment (Cores 201-1226B-2H and 201-1226E-1H), from intermediate depths near 100, 200, 310, and 380 mbsf (Cores 201-1226B-12H, 22H, 34X, and 43X), and from the deepest core at 420 mbsf (Core 201-1226B-47X) (Fig. F5). An additional sample for manganese reducers and other cultivations was taken near 300 mbsf (Core 201-1226E-18H).
Starting from Core 201-1226B-30X (281.5 mbsf), where coring switched from APC to XCB, core quality deteriorated because of high levels of seawater infiltration, which gave the sediment a mushy consistency. Elevated levels of perfluorocarbon (PFC) contamination were found in the disturbed portions of XCB cores, for example in Cores 201-1226B-30X and 40X (Table T8). In such cases, the remaining portions of consolidated sediment ("biscuits") were collected for a reduced sampling program that was improvised under the circumstances (see "Biscuit Sampling"). The full sampling program resumed for Cores 201-1226B-45X, 46X, and 47X at the bottom of Hole 1226B.
Microbiological sampling in Hole 1226E focused on several additional depths to complement the microbiological profiles obtained in Hole 1226B and to fill sampling gaps (Fig. F6). The mudline core of Hole 1226E was sampled at three depths (in Sections 201-1226E-1H-1, 1H-3, and 1H-5) to obtain finer resolution near the sediment surface, where the steepest biogeochemical gradients had been encountered during ODP Leg 138 and confirmed by interstitial water analyses from Hole 1226B (Fig. F4). An additional sample for cultivation of manganese-reducing bacteria in Hole 1226E at 300 mbsf was chosen according to the analyzed manganese profile of Hole 1226E, which showed a conspicuous manganese peak at that depth (Fig. F4).
In Hole 1226E, a continuous APC sample series focused on the layers below 260 mbsf that had been sampled by XCB coring in Hole 1226B. Starting with Core 201-1226E-16H, every second core was sampled according to the full program sampling scheme down to 326 mbsf (Fig. F6).
To explore the composition of microbial communities associated with oceanic crust and their contribution to basalt weathering, XCB drilling into basement rock was performed (Core 201-1226B-47X; 421.4 mbsf). The core catcher yielded fragments of basaltic rock that were sampled separately using a specially designed sample processing scheme (see "Rock Sampling and Distribution").
Samples of 1-cm3 plugs for prokaryote enumeration were taken on the catwalk from a total of 36 core sections at depths between 0.01 and 7.3 mbsf in Hole 1226E (4 samples) and between 8.85 and 420 mbsf in Hole 1226B (32 samples). Additionally, 2-mL samples or 25% slurry were taken from five slurries prepared in the laboratory (see "Total Prokaryotic Cell Counts" in "Procedures and Protocols" in "Microbiology" in the "Explanatory Notes" chapter). Slurries were made from Cores 201-1226B-2H, 12H, 22H, 43X, and 47X and from Core 201-1226E-1H.
Prokaryotes were present in 24 samples from the near surface to 419.4 mbsf (Fig. F7). Prokaryotes were not detected in the lowest sample at 420 mbsf. The highest number of prokaryotic cells was found in the near-surface sample (201-1226E-1H-1, 0-1 cm), which contained 4.63 x 108 cells/cm3. The overall depth profile of cell numbers per cubic centimeter follows a trend observed at other ODP sites (Parkes et al., 1994); however, cell numbers are substantially lower than the average previously observed trend in the upper few meters and remain lower than average to 40 mbsf (Fig. F8). The profile then conforms well from ~40 mbsf to near basement at 419.4 mbsf, except for an additional reduction in cell numbers between 90 and 170 mbsf. All data fit within 2  of the average previously observed trend over the depths between 8.9 mbsf (Sample 201-1226B-2H-3, 145-150 cm) and 419.4 mbsf (Sample 47X-2, 145-150 cm).
of the average previously observed trend over the depths between 8.9 mbsf (Sample 201-1226B-2H-3, 145-150 cm) and 419.4 mbsf (Sample 47X-2, 145-150 cm).
Numbers of dividing cells (suggested as an index of growth) are typically <10% of the total count. As expected, dividing cells as a percentage of the total count are high near the surface (Fig. F7) and decrease to a minimum at 113 mbsf (Sample 201-1226B-13H-3, 145-150 cm). The percentage of dividing cells then increases until 295.3 mbsf (Sample 201-1226B-32X-3, 145-150 cm), where 13.4% of the total was observed to be dividing. Below 320 mbsf, three of the five measurements did not detect any dividing cells. However, there are indications of a second peak in dividing cells between 368.4 and 381 mbsf (Samples 201-1226B-40X-1, 29-33 cm, and 43X-3, 145-150 cm).
Total counts were performed on five of the six slurry samples. The prokaryotic populations present in laboratory slurries were compared to those in adjacent plug samples taken for total counts using a two-way analysis of variance with interaction. There was no significant difference between the samples (F-value = 0.56; degrees of freedom = 1 and 14; interaction not significant).
While drilling cores for microbiology, the potential for contamination with bacteria from the surface is highly critical. Contamination tests were continuously conducted using solutes (PFT) or bacterial-sized particles (fluorescent microspheres) to check for the potential intrusion of drilling water from the periphery toward the center of cores and thus to confirm the suitability of the core material for microbiological research. We used the chemical and particle tracer techniques described in ODP Technical Note 28 (Smith et al., 2000). Furthermore, the freshly collected cores were visually examined for possible cracks and other signs of disturbance by observation through the transparent core liner. Core sections observed to be disturbed before or after subsampling were not analyzed further. Such disturbance phenomena are critical to the integrity of the core material and therefore also to its usefulness for microbiological studies.
The PFC tracer (see "Perfluorocarbon Tracer Contamination Tests" in "Procedures and Protocols" in "Microbiology" in the "Explanatory Notes" chapter) was injected continuously into the drilling fluid during drilling of Holes 1226B and 1226E. With a few exceptions, 5-cm3 subcores were taken from the bottom cut of each microbiology (MBIO) section or from the adjacent top of the immediately underlying section, as well as from 5-mL aliquots of each master slurry for contamination monitoring. In addition, samples from Section 201-1226B-47X-CC were collected to measure the concentration of PFT.
In all cases where PFT concentrations were below the detection limit, the delivery of PFT to the drill bit was positively confirmed by measuring the concentration of PFT in drilling fluid removed from the top of the core or by detection of PFT in sediment smeared along the edge of the core. At Site 1226, careful catwalk sampling and revised gas chromatography (GC) protocols (see "Microbiology" in the "Explanatory Notes" chapter and House et al., this volume) resulted in lower detection limits (~0.02 µL seawater contamination/g sediment or ~0.025 µL seawater contamination/mL slurry) than those at Site 1225.
Given these detection limits, the results (Table T8) indicate very low levels of surface seawater contamination for APC subcores, with most having contamination levels of ~0.04 µL seawater drilling fluid/g sediment. Of the 21 APC cores sampled, only two had PFT concentrations indicating seawater contamination of >0.1 µL seawater/g sediment. XCB cores were generally more contaminated. PFT concentrations in subcores taken on the catwalk from XCB cores had as little as 0.03 µL seawater/g sediment (Section 201-1226B-43X-4) or as much as 4.08 µL seawater/g sediment (Section 201-1226B-30X-4), with one-half of the XCB catwalk subcores showing 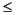 0.29 µL seawater/g sediment. The mean level of seawater contamination observed for subsamples taken from within intact biscuits of sediment from an XCB core was 0.24 µL seawater/g sediment. This was also close to the level of seawater contamination observed in a subcore of a 4-cm biscuit removed from Core 201-1226B-43X, broken open, and carefully subsampled for PFT analysis (Fig. F9). Two samples taken from the core catcher of Core 201-1226B-47X were analyzed for PFT concentration. The first was a piece of breccia that showed a high degree of seawater contamination (~1.8 µL seawater/g sediment), and the second was the crushed internal part of a carefully washed and sterilized rock sample. In contrast to the piece of breccia, the internal part of the washed and sterilized sample showed low levels of PFT, representing ~0.05 µL seawater/g sediment. This finding demonstrates that careful subsampling of hard rock material from the core catcher can result in microbiologically clean samples. Of the five master slurry samples taken from Site 1226, only the slurry from Core 210-1226B-2H showed a moderate concentration of PFT (representing ~0.3 µL seawater/mL slurry), whereas PFT concentration was below the detection limit in the others (Table T9). Finally, the PFT concentrations in three samples of interstitial water taken from XCB cores were analyzed. These samples showed higher levels of PFT than microbiological samples from the same cores, with an average contamination of ~0.5 µL seawater/mL interstitial water. However, the increased level of PFT in these samples may be a result of handling of samples during interstitial water pressing rather than a result of contamination by drilling fluid.
0.29 µL seawater/g sediment. The mean level of seawater contamination observed for subsamples taken from within intact biscuits of sediment from an XCB core was 0.24 µL seawater/g sediment. This was also close to the level of seawater contamination observed in a subcore of a 4-cm biscuit removed from Core 201-1226B-43X, broken open, and carefully subsampled for PFT analysis (Fig. F9). Two samples taken from the core catcher of Core 201-1226B-47X were analyzed for PFT concentration. The first was a piece of breccia that showed a high degree of seawater contamination (~1.8 µL seawater/g sediment), and the second was the crushed internal part of a carefully washed and sterilized rock sample. In contrast to the piece of breccia, the internal part of the washed and sterilized sample showed low levels of PFT, representing ~0.05 µL seawater/g sediment. This finding demonstrates that careful subsampling of hard rock material from the core catcher can result in microbiologically clean samples. Of the five master slurry samples taken from Site 1226, only the slurry from Core 210-1226B-2H showed a moderate concentration of PFT (representing ~0.3 µL seawater/mL slurry), whereas PFT concentration was below the detection limit in the others (Table T9). Finally, the PFT concentrations in three samples of interstitial water taken from XCB cores were analyzed. These samples showed higher levels of PFT than microbiological samples from the same cores, with an average contamination of ~0.5 µL seawater/mL interstitial water. However, the increased level of PFT in these samples may be a result of handling of samples during interstitial water pressing rather than a result of contamination by drilling fluid.
Background levels of PFT in various laboratories showed between undetectable levels and up to 9 x 10-12 g/mL air. Because of the high variability, no adjustment was made for laboratory air PFT contamination for these interstitial water samples. However, very high air levels of PFT were found in samples taken from the cold room (1.8 x 10-10 g/mL air). To avoid background problems, fresh samples were either carried from the laboratories onto the catwalk and capped in the open air, which had repeatedly tested PFT negative, or they were corrected for background readings of laboratory air. All values in the Table T8 are corrected for background.
Assuming 5 x 108 prokaryotic cells/L surface seawater, each 0.1 µL of seawater contamination may represent as many as 50 contaminating cells, if the sediment is porous enough to allow cells to travel with the PFT. However, the only slurry with detectable amounts of PFT (prepared from Core 201-1226B-2H) did not contain beads, indicating that prokaryotic cell mimics were not entrained into the sediment samples. Beads appear in this case to be a more realistic tracer of prokaryotic cells (see below).
Fluorescent beads (5 x 1011 beads/core) were deployed on all five cores from which slurries were subsequently made in the laboratory. Only on the last core run for slurries (Core 201-1226E-1H) did the bag with bead suspension fail to burst and beads were not deployed. For each slurry, three subsamples were processed: (1) a sample of the slurry, (2) a sample tenfold diluted in 2% formaldehyde (used also for direct cell counts), and (3) a scraping from the outer surface of the core (processed to confirm deployment of beads).
Beads were detected in three of the slurries (Table T10). However, in the first slurry (Section 201-1226E-1H-1) this was due to a procedural error during processing. Absence of beads was confirmed from the vial sample. For the other two positive slurry samples (Sections 201-1226B-22H-3 and 43X-2), only a single bead was detected and it is believed this was probably due to sample handling rather than contamination. The counting procedure was modified at this site by viewing the filter membrane under a 63x objective rather than a 100x objective. This had the effect of increasing coverage of the filter membrane from ~9,000 µm2/field of view to 22,800 µm2/field of view, so raising the sensitivity by a factor of 2.5. The eyepiece counting grid covers approximately one-third of a field of view. For both the samples above, only a single bead was found after thorough searching. In both cases, processing the vial sample indicated a negative result. This finding suggests that handling of the bead samples rather than contamination during coring was the problem. Handling protocols were further improved for subsequent sites.
Slurries for cultivation were usually prepared by subcoring with two 60-mL syringes from the center of two freshly broken surfaces (after precutting the core liner with the ODP cutter). This represented a change in method from Site 1225 (see "Cultivations" in "Microbiology" in the "Site 1225" chapter and "Cultivation of Microorganisms" in "Procedures and Protocols" in "Microbiology" in the "Explanatory Notes" chapter). From Sections 201-1226B-43X-3 and 47X-1, slurries were prepared by breaking cleaned biscuits (see "Biscuit Sampling" below) and removing material from the inner untouched surfaces with sterile scalpels.
All MPN dilutions and enrichments inoculated using samples from Site 1226 are listed in Table T11. A strong indication for manganese(IV) reduction was given by the dissolved manganese interstitial water profile, which showed pronounced peaks of dissolved manganese close to the sediment surface and at 300 mbsf (see Fig. F4). At 350 mbsf the dissolved manganese concentrations show a minimum and increase toward the bottom of the hole to concentrations that are even higher than at the surface of the sediment. Consequently, MPN enrichments of manganese(IV)-reducing prokaryotes focused on this interval.
After the first few days of incubation, there was growth of rod-shaped cells in one enrichment assay at 25°C (room temperature) on medium 201-3 (see Table T5 in the "Explanatory Notes" chapter), designed for anaerobic heterotrophs with nitrate as the electron acceptor. The culture was inoculated with sediment from Section 201-1226B-34X-3. This first positive result needs to be confirmed by further subculturing and by verifying with molecular data. Furthermore, the potential for previous contamination of the samples used for enrichment must be considered (see "Contamination Tests").
13C substrate incubations were initiated for postcruise analysis by FISH-secondary ion mass spectrometry (SIMS) using material from Cores 201-1226B-2H and 12H. In this case, 10 mL of the master slurry was injected into each bottle. The 13C substrates used were methane, acetate, or glucose. However, for Core 201-1226B-12H, the 13C acetate bottle had nonlabeled methane in the headspace.
Sediment cores recovered by XCB commonly had a mushy consistency because of mechanical disturbance and infiltration of drilling fluid. Such cores contained relatively few undisturbed portions, referred to in ODP parlance as biscuits. A biscuit is a hard, round to ellipsoidal or subcylindrical clump of sediment (3-7 cm in diameter, occasionally as long as 15 cm in length) that fails to break with hand pressure between the fingers and thumb (Fig. F9). Biscuits that fractured along planes with hand pressure were discarded because wet spots were commonly present along the fracture surfaces and were shown by PFT analysis to be contaminated (see "Contamination Tests"). In Hole 1226B, Cores 201-1226B-30X and deeper were recovered as XCB cores. Mushy sediment consistency was noticed in cores and sections deeper than Section 201-1226B-32X-3. Less than 10% of the sediment volume was recovered as usable biscuits in Cores 201-1226B-33X through 35X. From these and subsequent cores, biscuits were recovered, kept under nitrogen for 1-4 hr, and sampled according to the following scheme:
Cores 201-1226B-45X and 46X exhibited no mushiness and biscuit formation and were cut and subsampled as described in "Core Handling and Sampling" in "Introduction and Background" in "Microbiology" in the "Explanatory Notes" chapter and in Figure F5. With Core 201-1226B-47X, the biscuit sampling protocol was used for slurry preparation, whereas other samples were taken by the routine procedures.
Several individual pieces of rock of various sizes as large as 4.5 cm (dark gray to black basalt) were obtained from the core catcher of the lowermost core (201-1226B-47X). Furthermore, a large round rock piece was obtained that initially appeared to be a solid core sample of ~8 cm in length but broke into two smaller pieces during cleaning and investigation. These pieces were grayish green and contained pieces of gray-black weathered basalt (up to ~3 cm in length). A few of these weathered basalt fragments contained small red inclusions (tentatively identified as hematite). The cracks in the basalt were filled with white crystalline material that was visible when examined with a dissecting microscope. The white material is assumed to include calcite, as some could be removed with 3-M HCl treatment. Further examination suggested that the large core piece contained coring debris around a natural conglomerate (breccia). With a sterile spatula, the two pieces were broken into successively smaller pieces to obtain fresh uncontaminated surfaces from which material (16.6 g) for inoculations was obtained with a sterile scalpel. Some of the softer black material could be shaved off with a scalpel. Material from the inside of a freshly broken surface (3 g) showed a high concentration of the PFT tracer, indicating seawater contamination (Table T8). However, the same material was found to be free of fluorescent beads. Softer, partly creamy material from the outer rim (after scratching off the outer 2 mm), was obtained as control material (drilling fluid-contaminated inocula). The following enrichment media (see Table T5 in the "Explanatory Notes" chapter) were inoculated with ~1 g of the inner rock or, as a control, with the outer rim material: H2/HCO3-, H2/HCO3-/Fe(III), and a mixture of FERM-gluc-8.0 and FERM-xyl-8.0. Except for the H2/HCO3-/Fe(III) media, one tube each was prepared for anaerobic or for aerobic (headspace of air:nitrogen = 1:3) incubations. One set was incubated at 60°C and the other set at room temperature (20°-26°C). Pieces broken off from inside of the rock and some shaved-off material were kept in sterile nitrogen-flushed glass vials at 4°C for postcruise molecular work and further enrichments.
One piece of basalt from Section 201-1226D-47X-CC (Fig. F10) was washed with sterile nitrogen-flushed marine salts solution, crushed in the sterile shipboard rock crusher (see "Rock Sampling" in "Procedures and Protocols" in "Microbiology" in the "Explanatory Notes" chapter), and used for inoculation of various culture media. The wash water from the sample had the consistency of a diluted sediment slurry (~250 mL with 5% sediment) and was used as inoculum for controls and for testing for the presence of contaminating surface microbes.
The following enrichments were initiated:
In addition, 3.2 g of the HCl-washed and crushed material was stored at 4°C and will be used for postcruise analysis including scanning electron microscopy, electron probe microanalyzer, and XRD analyses.
One piece of basalt was only washed several times with sterile saline solution and then used directly for enrichment of anaerobic iron(III) and manganese(IV) reducers at room temperature. Several of the blackish rock pieces (either directly from the core catcher or from the crumbling piece of breccia) were kept for postcruise experiments. Two pieces (washed in ethanol:phosphate buffered saline [PBS] and stored at -20°C) will be analyzed by FISH-SIMS (see "Sample Preparation for FISH-SIMS" in "Molecular Analysis" in "Procedures and Protocols" in "Microbiology" in the "Explanatory Notes" chapter).