 15N of Pore Water Ammonium and Sedimentary Organic Matter
15N of Pore Water Ammonium and Sedimentary Organic Matter
Site 1230 is located on the lower slope of the Peru Trench (9°6.7525´S, 80°35.0100´W) within 100 m of the location of ODP Leg 112 Site 685 (Fig. F1). Water depth at this site is 5086 m, but biogeochemical processes observed in the sediments are more typical of an ocean margin than a deep-sea setting (D'Hondt, Jørgensen, Miller, et al., 2003; Suess, von Huene, et al., 1988). The sediments at Site 1230 were divided into two lithostratigraphic units. Unit I (0–215.8 meters below seafloor [mbsf]) is Holocene–Pleistocene in age and consists of biogenic sediments mixed with siliciclastic components (D'Hondt, Jørgensen, Miller, et al., 2003). The sequence is characterized by alternating layers of diatom-nannofossil ooze and marl with dark gray to olive clays. Graded layers were presumably deposited by turbidite events that originated most likely near the shelf break and incorporated material from intermediate depths along the way (Suess, von Huene, et al., 1988). Unit II (215.8–278.3 mbsf) was deposited during the Miocene and is separated from Unit I by an unconformity. The presence of a fractured layer at this horizon suggests the unconformity is of tectonic origin. Sediments consist of diatom- and silt-rich deposits interbedded with clay rich layers. Estimated sediment accumulation rates for Quaternary and Miocene sequences are 100 m/m.y. and 200 m/m.y., respectively (Suess, von Huene, et al., 1988).
Concentrations of both dissolved inorganic carbon (DIC) and ammonium, the major metabolites of microbial decomposition of organic matter, are exceptionally high at this site. Concentrations of DIC reach a maximum of 162 mM at 132 mbsf, decreasing to ~130 mM below 200 mbsf. Ammonium concentrations reach a maximum value of 38 mM at about the same depth as the DIC maximum; similar to DIC concentrations, they decrease below 132 mbsf to ~25 mM at the bottom of the measured profile at 250 mbsf (D'Hondt, Jørgensen, Miller, et al., 2003).
Sulfate is depleted below the detection limit within the upper 7 mbsf, and dissolved methane concentrations increase steeply below this horizon, reaching values up to 7 mM at 17 mbsf. The sediments at this site also contain methane hydrates (Suess, von Huene, et al., 1988; D'Hondt, Jørgensen, Miller, et al., 2003).
 15N of Pore Water Ammonium and Sedimentary Organic Matter
15N of Pore Water Ammonium and Sedimentary Organic Matter
TOC concentrations at Site 1230 have moderate values of ~2 wt% (TOC measurements, details, and results are presented in
Meister et al., this volume), and TN ranges between 0.3 and 0.4 wt% (Fig. F2). Calculations using experimentally derived values for ammonium distribution coefficient (Prokopenko, 2004) indicate that adsorbed ammonium constitutes no more than 10% of TN values (the values vary with porosity and pore water ammonium concentrations). Atomic C/N ratios are between 9 and 11 and are relatively constant through the whole sediment column (Table T1). Nitrogen isotopic ratios of the sediments vary between 5 and 7.5
and 7.5 (average value = 5.71
(average value = 5.71 ± 0.19
± 0.19 ) and do not show any significant change with depth (Fig. F3). Three horizons at 47, 72, and 100 mbsf have higher
) and do not show any significant change with depth (Fig. F3). Three horizons at 47, 72, and 100 mbsf have higher  15N values of 7.5
15N values of 7.5 –8
–8 .
.
The isotopic composition of ammonium is relatively constant through the whole profile, with most values between 4.6 and 5.9
and 5.9 (Fig. F2). The average
(Fig. F2). The average  15N of pore water ammonium at this site is 5.0
15N of pore water ammonium at this site is 5.0 ± 0.1
± 0.1 , which is ~0.7
, which is ~0.7 ± 0.2
± 0.2 lighter than the average
lighter than the average  15N of sediments.
15N of sediments.
The ammonium and DIC profiles indicate that the labile organic matter in Unit I is decomposed as it is buried, releasing both metabolites. The maximum DIC and ammonium concentrations create downward diffusion into Unit II, which apparently had lower solute concentrations of both metabolites before its tectonic juxtaposition with Unit I. Both units have organic matter with similar  15N, so diffusion of ammonium across the unconformity should not strongly influence the
15N, so diffusion of ammonium across the unconformity should not strongly influence the  15N of ammonium. Indeed, our measurements showed no change in
15N of ammonium. Indeed, our measurements showed no change in  15N of pore water ammonium across the boundary.
15N of pore water ammonium across the boundary.
Because ammonium is the product of the organic matter decomposition, we can calculate the amount of organic nitrogen (Norg) lost to degradation as a function of depth based on the amount of ammonium released through a sediment interval. The approach is based on flux calculations at the upper and lower boundaries of Unit I and estimates nitrogen loss for all of Unit I. In this approach, we determined tangents to the ammonium profile near the top (1.35 mbsf) of the sediment column and near the ammonium maximum (119.47 mbsf). We used these tangents to calculate diffusive fluxes based on Fick's first law. The advective contribution to the total ammonium flux was deemed unimportant (Peclet number = 3) (Table T2). Therefore, advective flux was not included in the calculations. The calculated difference of 0.98 ± 0.20 µmol/(cm2yr) represents the net nitrogen released from solid phases through this interval. The present sedimentation flux of organic nitrogen was calculated using the average sediment accumulation rate of 100 m/m.y. (D'Hondt, Jørgensen, Miller, et al., 2003), average porosity of 0.75, and nitrogen in the topmost interval of 0.37 wt%. The Norg sedimentation flux at 1.35 mbsf is 1.59 ± 0.30 µmol/(cm2yr). Thus, the ammonium production flux indicates that ~60% ± 15% of Norg being deposited at 1.35 mbsf is decomposed and lost as ammonium. However, it is likely that flux at the 119.47-mbsf horizon is not quite in a steady state due to currently occurring diffusion of ammonium into the Layer II of Miocene age. Consequently, 60% loss of Norg via decomposition may be just a minimum estimate of the amount of Norg lost. The average isotopic composition of ammonium is 0.7 ± 0.2
± 0.2 lighter than the
lighter than the  15N of Norg. One possible explanation for this difference may be a small contribution from dissolved organic nitrogen (DON) in the pore water, if it degraded during the ammonium extraction procedure (Sigman, 1997). Dissolved organic carbon (DOC) at this site reaches concentrations of 20 mM (Smith, this volume). DON concentrations were not measured during Leg 201, but the contribution from DON is probably minor (D. Smith, pers. comm., 2004).
15N of Norg. One possible explanation for this difference may be a small contribution from dissolved organic nitrogen (DON) in the pore water, if it degraded during the ammonium extraction procedure (Sigman, 1997). Dissolved organic carbon (DOC) at this site reaches concentrations of 20 mM (Smith, this volume). DON concentrations were not measured during Leg 201, but the contribution from DON is probably minor (D. Smith, pers. comm., 2004).
Another possibility is that the difference is a result of fractionation associated with release of ammonium from decomposing organic matter with a fractionation factor, 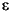 = –0.7
= –0.7 ± 0.2
± 0.2 . Despite the fact that at least half of organic nitrogen has been lost to diagenesis between 1.35 and 119.47 mbsf, there is no pronounced change in the isotopic composition of the sediments with depth. If the small isotopic enrichment of ammonium is indeed due to fractionation during ammonium release, the expected change in the solid-phase
. Despite the fact that at least half of organic nitrogen has been lost to diagenesis between 1.35 and 119.47 mbsf, there is no pronounced change in the isotopic composition of the sediments with depth. If the small isotopic enrichment of ammonium is indeed due to fractionation during ammonium release, the expected change in the solid-phase  15N would have been negligibly small, ~0.7
15N would have been negligibly small, ~0.7 . The absence of depth dependency of
. The absence of depth dependency of  15N of the sedimentary nitrogen and close similarity between
15N of the sedimentary nitrogen and close similarity between  15N of ammonium solid-phase N both suggest that at Site 1230 little isotopic fractionation is associated with long-term diagenesis of sedimentary organic matter. One additional implication is that the
15N of ammonium solid-phase N both suggest that at Site 1230 little isotopic fractionation is associated with long-term diagenesis of sedimentary organic matter. One additional implication is that the  15N of material reaching the sediments at this site has been rather constant through time.
15N of material reaching the sediments at this site has been rather constant through time.