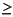 95 vol%) from 23.1 to 121.8 mcd. No significant amounts of higher molecular weight hydrocarbons were observed (Table T16).
95 vol%) from 23.1 to 121.8 mcd. No significant amounts of higher molecular weight hydrocarbons were observed (Table T16).
Concentrations of headspace and vacutainer gases were routinely monitored in Hole 1233B sediments according to shipboard safety and pollution prevention considerations. Low methane concentrations were first detected in the shallowest headspace gas sample at 1.5 mcd (Table T16; Fig. F24). Methane concentrations increased by 21.1 mcd, and vacutainer samples had high methane concentrations (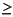 95 vol%) from 23.1 to 121.8 mcd. No significant amounts of higher molecular weight hydrocarbons were observed (Table T16).
95 vol%) from 23.1 to 121.8 mcd. No significant amounts of higher molecular weight hydrocarbons were observed (Table T16).
High methane concentrations, low concentrations of higher molecular weight hydrocarbons, and the resulting high C1/C2 ratio (Fig. F24) indicate that the methane originates from in situ fermentation (methanogenesis) of sedimentary organic matter. A biogenic origin for methane is supported by the disappearance of interstitial dissolved sulfate below 2 mcd. The presence of interstitial sulfate inhibits methanogenesis in marine sediments (Claypool and Kvenvolden, 1983).
We collected 14 interstitial water samples from Site 1233, 11 from Hole 1233B and 3 from Hole 1233C. These are treated as constituting a single mcd profile. Chemical gradients at this site (Table T17) reflect the likely presence of gas hydrates, the influence of organic matter diagenesis by microbially mediated oxidation reactions, a limited degree of biogenic opal dissolution, and the effects of authigenic mineralization reactions on fluid composition.
Chlorinity decreases by >12%, from 561 mM at 1.5 mcd to ~490 mM by 110-120 mcd (Fig. F25). Salinity, measured refractively as total dissolved solids, ranges from 37 to 30, decreasing with increasing depth (Table T17). Sodium concentrations measured by inductively coupled plasma-atomic emission spectrophotometry were typically within <4% of those estimated by charge balance reported here (Table T17). Sodium concentrations decrease by >12% by 110-120 mcd, in accord with the decreases seen in chlorinity and salinity.
The decreasing chlorinity gradient at Site 1233 is significantly larger than the gradients observed at the deeper-water, mid-slope Peru margin basin sites drilled during Leg 112 (Sites 682, 683, 685, and 688; Suess, von Huene, et al., 1988). The decomposition of gas hydrates in Site 1233 sediments can explain the observed chloride gradient in interstitial water as a result of dilution, either through in situ decomposition at depth in the sediment column or during sediment recovery. The second process is usually evident from spiky, low values in chloride profiles, not observed here. Other possible explanations for the chloride gradient include mineral dehydration reactions at depth, clay membrane ion filtration reactions at depth, and advection of fresher fluid at depth (Kastner et al., 1990).
Organic matter diagenesis, driven by microbial oxidation reactions, dominates many of the interstitial water profiles. The relatively low organic carbon contents are apparently counterbalanced by high total sedimentation rates to result in pronounced depth variations in interstitial water chemistry, as typically observed in more organic carbon-rich, continental margin settings. Sulfate concentration at 1.5 mcd is 12.5 mM, already substantially lower than typical seawater values of 29 mM, and all deeper samples have sulfate concentrations below the detection limit (~0.5 mM). Organic matter decomposition by sulfate reduction and methanogenesis drives large increases in alkalinity, which increases to peak values of >60 mM from 21.0 to 44.3 mcd then decreases to 30 mM at 121.8 mcd.
The profiles of the reduced forms of the secondary oxidants manganese and iron show variations with depth, although not simply related to that of sulfate. Dissolved manganese concentrations average <3 然, with small maxima at 11.0 mcd and from 76.9 to 87.5 mcd. Dissolved iron concentrations rapidly increase to 53 然 at 11.0 mcd, decrease sharply to <15 然 by 21.0 mcd, and then decrease gradually with increasing depth.
Organic matter decomposition generates increases in phosphate and ammonium in interstitial water. Phosphate concentrations are >100 然 throughout, with values >210 然 from 11.0 to 66.7 mcd. Ammonium concentrations increase from 1.7 mM at 1.5 mcd to values >5 mM from 11.0 to 76.9 mcd, with decreasing values to 3.1 mM at 121.8 mcd. The maximum in ammonium concentration is broader and deeper than the phosphate maxima.
Dissolved silicate concentrations average ~570 然 (Fig. F25), indicating that the interstitial waters are not at saturation with respect to biogenic opal. This may reflect the limited amount of biogenic opal available for dissolution or other controls on opal solubility in these sediments. Barium concentrations increase to a sharp maximum of 36 然 at 4.5 mcd, coincident with the disappearance of dissolved sulfate, and are <10 然 from 21.0 mcd to total depth. The dissolution of barite, driven by the decrease in dissolved sulfate, is consistent with the observed profiles. Boron concentrations increase to values >800 然 from 11.0 to 66.7 mcd then generally decrease with increasing depth to values at low as ~600 然. The resemblance of the boron and ammonium profiles indicates that adsorption/desorption reactions may influence the boron profile.
Calcium concentrations decrease sharply from 6 mM at 1.5 mcd to values <2 mM from 4.5 to 11.0 mcd, then increase to ~3 mM at 66.7 mcd, followed by a decrease with increasing depth to a minimum of 0.7 mM at 98.1 mcd. Magnesium concentrations increase to >55 mM from 11.0 to 54.7 mcd, then decrease to 43 mM at 121.8 mcd. The very strong decrease in calcium by 4.5 mcd is consistent with authigenic mineralization reactions driven by the alkalinity increase. Increasing magnesium/calcium ratios with the calcium decrease indicate that calcite precipitation is the most likely reaction taking place. Magnesium/calcium ratios are >25 from 4.5 to 32.6 mcd, decreasing to only as low as 19, before increasing to a sharp maximum of 69 at 98.1 mcd coincident with the calcium minimum. Lithium concentrations first decrease sharply then generally increase with increasing depth. Strontium concentrations generally decrease slightly with increasing depth, and potassium concentrations increase to >12 mM from 11.0 to 44.3 mcd then decrease with increasing depth (Fig. F25).
Inorganic carbon (IC), total carbon (TC), and total nitrogen (TN) concentrations were determined on sediment samples from Holes 1233A, 1233C, and 1233E (Table T18). Organic matter carbon/nitrogen ratios were employed to characterize the organic matter.
The calcium carbonate profile reflects the overall uniformity of the sedimentary section at this site (Fig. F26; also see "Lithostratigraphy"). Calcium carbonate concentrations are low throughout the record, ranging between 1.2 and 10.8 wt% (average = 5.4 wt%). Calcium carbonate concentrations are higher in the uppermost 30 mcd, with lower concentrations in the intervals from 30 to 60 mcd and from 102 to 126 mcd. Stronger-amplitude variations of 3-6 wt% mark the interval from 80 to 105 mcd. The highest calcium carbonate concentrations correspond to observations of increased abundance of calcareous nannofossils and foraminifers (see "Lithostratigraphy").
TOC concentrations range between 0.4 and 2.4 wt% (average = 0.9 wt%) (Table T18). TOC variations are similar to those of calcium carbonate (Fig. F26). TOC concentrations decrease from ~2 wt% at the top to ~0.5 wt% at 30 mcd. At greater depths, the TOC concentrations and amplitude fluctuations remain low. TN concentrations have similar trends.
TOC/TN ratios are between 5 and 9 (average = 5.9) (Table T18), indicating a predominance of marine organic matter throughout the record (Bordovskiy, 1965; Emerson and Hedges, 1988; Meyers, 1997). In addition, the lack of a significant correlation between TOC/TN ratios and decreases in TOC contents indicates that the TOC decrease in the upper 30 mcd is not induced by diagenesis of the organic matter (Fig. F27). This decrease in TOC and, to a lesser extent, in calcium carbonate concentrations may reflect a decrease in export productivity and/or a dilution effect caused by an increased supply of siliciclastic material (see "Lithostratigraphy").