GEOCHEMISTRY
Concentrations of headspace gases were routinely monitored in Hole 1238A sediments according to shipboard safety and pollution prevention considerations. Very low amounts of methane were first detected in a headspace gas sample at 10.1 mcd (Fig. F30; Table T13). At greater depths, methane increased smoothly, reaching a maximum of >60 ppmv (and always <100 ppmv) from 167.9 to 209.0 mcd. Methane concentrations decreased downhole to ~10 ppmv at the bottom of the hole. Very small amounts of ethane were detected, and no detectable higher molecular weight hydrocarbons were observed. Low methane concentrations indicate that this gas probably originates from in situ fermentation (methanogenesis) of the organic matter buried in the sediments (Claypool and Kvenvolden, 1983).
Interstitial Water Geochemistry
We collected 33 interstitial water samples from Hole 1238A. Chemical gradients at this site (Table T14; Fig. F31) reflect the influence of organic matter oxidation, the dissolution of biogenic silica and its reprecipitation in authigenic phases, the effects of authigenic calcite precipitation, and the diffusive influence of basalt alteration processes. The dissolved silicate profile shows a pronounced difference from lithologic Subunit IA to Subunit IB.
Chlorinity increases from 552 mM at 3.0 mcd to >560 mM from 32.3 to 301.7 mcd then decreases to 544 mM at 457.9 mcd (Fig. F31). Salinity, measured refractively as total dissolved solids, ranges from 32 to 34 (Table T14). Sodium concentrations measured by inductively coupled plasma-atomic emission spectrophotometry averaged 1% lower than those estimated by charge balance reported here (Table T14). Sodium concentrations parallel chlorinity, with a total range from 468 to 492 mM.
Organic matter diagenesis, driven by microbially mediated oxidation reactions, influences the interstitial water chemistry. Sulfate reduction occurs but does not go to complete disappearance of sulfate, indicating that the rate of labile organic matter supply is not sufficient to exhaust the sulfate supply. Sulfate decreases from 26.8 mM at 3.0 mcd to generally <10 mM by 74.5 mcd, coincident with the top of the organic carbon-rich zone from 78.6 to 94.9 mcd. The relatively wide depth range of sulfate reduction resulted in noticeable hydrogen sulfide in interstitial water samples and in sediments on splitting. Sulfate values are lower in Subunit IB.
Alkalinity increases from 31 mM near the seafloor to >17 mM from 82.4 to 92.6 mcd, consistent with alkalinity generation from sulfate reduction, then declines to 6.9 mM at 386.3 mcd and to values as low as 2.3 mM at 457.9 mcd. The decline in upper sediments is primarily the result of authigenic carbonate precipitation, based on the calcium profile changes, whereas the deepest decline is also likely from the influence of basalt alteration reactions at depth.
Dissolved manganese has a sharp subsurface maximum up to 5.5 然 at 10.1 mcd, indicative of suboxic oxidation of organic carbon by manganese reduction, then decreases to consistently below the detection limit (0.01 然) by 64.3 mcd. Dissolved iron has a complex profile with depth, with multiple peaks >10-20 然.
Phosphate concentrations increase from 5.2 然 at 3.0 mcd to >15 然 from 9.5 to 64.3 mcd. A sharp 1-point maximum of 78 然 at 54.3 mcd is analytically robust but may be an artifact related to the acidification of the samples required to deal with the presence of hydrogen sulfide and/or the possible release of scavenged phosphate from fine particles. Ammonium concentrations increase from below the detection limit (0.4 mM) at 3.0 mcd to an average of 3 mM from 82.4 to 386.3 mcd, with a small decrease at the base of Subunit IB (Fig. F31). The increases in phosphate and ammonium result from the oxidation of organic matter.
Dissolved silicate increases from 668 然 at 3.0 mcd to >1800 然 from 344.1 to 365.2 mcd, with a small decrease in silicate concentration coincident with the depth interval over which silicification of the sediment was first noted (see "Postdepositional
Diagenesis" in "Lithostratigraphy"). The increase in dissolved silicate with increasing depth is consistent with temperature-controlled solubility for biogenic opal in this site. Silicate concentrations decrease markedly in Subunit IB to <1000 然 as a consequence of the formation of chert and its diagenetic predecessors observed in the sediment and log data.
Barium concentrations increase from 0.3 然 at 3.0 mcd to 0.8 然 at 386.3 mcd, with higher values in the base of Subunit IB with increasing sulfate depletion. This suggests that the dissolution of barite, driven by the decrease in sulfate, influences barium concentrations. Boron concentrations increase from 490 然 at 3.0 mcd to 836 然 at 386.3 mcd and then to >1000 然 from 436.3 to 457.9 mcd.
Calcium decreases from 10.0 mM at 3.0 mcd to 6.1 mM at 54.3 mcd, then increases to 16.3 mM at 386.3 mcd, with a steeper increase with depth in Subunit IB. The shallow part of this profile is controlled by authigenic calcite precipitation driven by the alkalinity increase, and the deeper portion reflects the diffusive influence of basalt alteration. Magnesium is dominated by the diffusive influence of basalt alteration reactions at depth, with Mg decreasing from 51.2 mM at 3.0 mcd to 30.3 mM at 386.3 mcd, followed by a steeper decline with depth in Subunit IB. Magnesium/calcium ratios increase from 5.1 at 3.0 mcd to 7.8 from 32.3 to 54.3 mcd, then decrease to 1.9 by 386.3 mcd and to values as low as 1.1-1.2 in the base of Subunit IB (Fig. F31). The increase in magnesium/calcium in the shallower sediments, driven by the decrease in calcium, indicates that calcite precipitation is the dominant authigenic mineralization reaction. Below this zone, the increase in calcium (~3.3 mM/100 m) is correlated to the decrease in magnesium (approximately -4.9 mM/100 m), consistent with control of these profiles by the diffusive influence of basalt alteration reactions.
Lithium concentrations increase from 26 然 at 3.0 mcd to 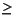 50 然 from 92.6 to 259.3 mcd then decrease to 31-32 然 from 436.3 to 457.9 mcd. The middepth maximum requires a source of lithium from the sediments, whereas basement alteration reactions at low temperatures are a sink for lithium. Strontium concentrations increase steadily from 87 然 at 3.0 mcd to >750 然 from 436.3 to 457.9 mcd. This profile does not resemble those dominated by the influence of biogenic calcite recrystallization at depths of tens to hundreds of meters but does indicate a source of dissolved strontium at depth at this site. Potassium decreases from 11.7 mM at 3.0 mcd to 6.2 mM at 457.9 mcd. Low-temperature basalt alteration reactions are a sink for dissolved potassium, and this profile can be explained by the diffusive influence of basalt alteration reactions.
50 然 from 92.6 to 259.3 mcd then decrease to 31-32 然 from 436.3 to 457.9 mcd. The middepth maximum requires a source of lithium from the sediments, whereas basement alteration reactions at low temperatures are a sink for lithium. Strontium concentrations increase steadily from 87 然 at 3.0 mcd to >750 然 from 436.3 to 457.9 mcd. This profile does not resemble those dominated by the influence of biogenic calcite recrystallization at depths of tens to hundreds of meters but does indicate a source of dissolved strontium at depth at this site. Potassium decreases from 11.7 mM at 3.0 mcd to 6.2 mM at 457.9 mcd. Low-temperature basalt alteration reactions are a sink for dissolved potassium, and this profile can be explained by the diffusive influence of basalt alteration reactions.
Sedimentary Inorganic Carbon, Organic Carbon, Nitrogen, Sulfur, and Uranium Concentrations
Inorganic carbon (IC), total carbon (TC), total nitrogen (TN), and total sulfur (TS) were determined on sediment samples from Hole 1238A (Table T15). Organic matter carbon/nitrogen ratios were employed to characterize the organic matter.
Calcium carbonate concentrations range between 17.4 and 94.3 wt% (average = 60.3 wt%) (Table T15; Fig. F32). Calcium carbonate concentrations increase gradually with depth with meter-scale variations as large as 10-20 wt% throughout. These variations may reflect the productivity of calcareous organisms relative to the siliceous organisms or changes in preservation. Today, the site lies well above the regional lysocline. However, significant local dissolution here may occur in response to organic carbon degradation. One reason for the long-term change in calcium carbonate may be the tectonic migration of the site toward more coastal conditions with a greater delivery of terrigenous material diluting biogenic components. Calcium carbonate concentrations are at a minimum, with values generally <60 wt% from 78.3 to 112.6 mcd. This large drop is associated with an increase in diatom abundance during this interval (see "Diatoms"
in "Biostratigraphy"). Again, the combined influences of production and dissolution may be responsible. At depths >400 mcd, some individual samples have low calcium carbonate concentrations.
TOC concentrations range between values near detection limit and 4.3 wt% (average = 0.9 wt%) (Table T15; Fig. F32). The TOC and calcium carbonate profiles correlate inversely. TOC concentrations show a general decrease with depth, with values decreasing from ~1.5 wt% in the upper 4 m to <0.1 wt% at the bottom and small variations of ~0.5 wt% throughout the record. TOC concentrations reach a maximum with values generally >1.5 wt% from 78.6 to 112.6 mcd, with the TOC maximum corresponding to the calcium carbonate minimum.
TOC/TN ratios vary from 3.4 to 11.8 (Table T15; Fig. F32). TOC/TN ratios average 7.1, a value typical of unaltered algal material (Bordovskiy, 1965; Emerson and Hedges, 1988; Meyers, 1997), and most of the samples have TOC/TN ratios <9 (Fig. F33). A large change in land-derived organic matter input is excluded, since TOC/TN variations are small compared to TOC content variations, for instance, in the interval where TOC is very high. Changes observed in the organic carbon contents should result from the combination of productivity, preservation of the organic matter, and dilution by calcium carbonate.
The redox conditions in the sediments at the time of deposition can be inferred from the variations in concentration of redox-sensitive components, such as sulfur and uranium. TS concentrations range between 0.1 and 1.4 wt% (average = 0.4 wt%) (Table T15). Higher TS contents are detected in the interval where TOC content is the highest. This could be attributed to the formation of pyrite during the microbial degradation of organic matter by sulfate reduction, possibly driven by an increase in labile organic matter input to the sediment. The uranium profile is derived from the natural gamma ray activity signal measured during downhole logging operations (see "Natural Gamma
Radiation" in "Downhole Measurements"). Uranium is a conservative element, soluble in seawater as U(VI) (unreactive uranyl carbonate complex) and insoluble when reduced to U(IV). Uranium is supplied to the pore water by diffusion from the overlying water. It precipitates as an authigenic phase (uraninite; UO2) at or just below the depth of iron oxide reduction during organic matter diagenesis (Barnes and Cochran, 1990; Klinkhammer and Palmer, 1991). Therefore, sedimentary uranium is a good indicator of suboxic conditions in the sediments. A comparison of U contents and TOC contents at Site 1238 shows a good correlation between the two records (see Fig. F42), indicating more reducing (suboxic) conditions in the sediments when (labile) organic matter input to the sediment increased. Again, this supports an interpretation that high concentrations of organic matter are driven by productivity rather than dilution or preservation.

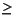 50 然 from 92.6 to 259.3 mcd then decrease to 31-32 然 from 436.3 to 457.9 mcd. The middepth maximum requires a source of lithium from the sediments, whereas basement alteration reactions at low temperatures are a sink for lithium. Strontium concentrations increase steadily from 87 然 at 3.0 mcd to >750 然 from 436.3 to 457.9 mcd. This profile does not resemble those dominated by the influence of biogenic calcite recrystallization at depths of tens to hundreds of meters but does indicate a source of dissolved strontium at depth at this site. Potassium decreases from 11.7 mM at 3.0 mcd to 6.2 mM at 457.9 mcd. Low-temperature basalt alteration reactions are a sink for dissolved potassium, and this profile can be explained by the diffusive influence of basalt alteration reactions.
50 然 from 92.6 to 259.3 mcd then decrease to 31-32 然 from 436.3 to 457.9 mcd. The middepth maximum requires a source of lithium from the sediments, whereas basement alteration reactions at low temperatures are a sink for lithium. Strontium concentrations increase steadily from 87 然 at 3.0 mcd to >750 然 from 436.3 to 457.9 mcd. This profile does not resemble those dominated by the influence of biogenic calcite recrystallization at depths of tens to hundreds of meters but does indicate a source of dissolved strontium at depth at this site. Potassium decreases from 11.7 mM at 3.0 mcd to 6.2 mM at 457.9 mcd. Low-temperature basalt alteration reactions are a sink for dissolved potassium, and this profile can be explained by the diffusive influence of basalt alteration reactions.