BIOGEOCHEMISTRY
The interstitial water (IW) sampling strategy for Site 1227 was designed to determine chemical profiles at high vertical resolution for comparison with Sites 1228 and 1229. The aim was to resolve the biogeochemical processes occurring in these deeply buried marine sediments where subsurface brines influence interstitial water chemistry. In Hole 1227A, IW samples were taken from each section of Cores 201-1227A-1H through 5H, with the exception of Core 4H (two samples), down to 42.95 mbsf. The sampling frequency was reduced to three IW samples in Core 201-1227A-6H and two IW samples per core below Core 6H down to the maximum penetration depth. Low recovery in Cores 201-1227A-14H and 16H allowed subsampling of only one IW sample from each. A total of 42 IW samples down to 144.45 mbsf were taken from Hole 1227A. An additional 18 IW samples collected from Hole 1227D targeted specific intervals of interest. One was the sediment/water interface, with five samples collected from 0.00 to 1.80 mbsf in Section 201-1227D-1H-1. Another was the sulfate-methane transition in Cores 201-1227D-4H and 5H from 27.85 to 42.91 mbsf (Table T2), with six samples collected from Core 4H and five from Core 5H. Analysis of this interval in Hole 1227A had shown the depletion of dissolved sulfate and the presence of elevated levels of dissolved methane and barium. In addition to routinely employed chemical analyses, we acquired IW concentration data for hydrogen, acetate, formate, total inorganic carbon, manganese, iron, and headspace methane.
Alkalinity and DIC have similar profiles downhole. Alkalinity increases steeply from 3.3 mM near the sediment/water interface to a maximum of ~24 mM at 41.5 mbsf, below which it decreases to ~22 mM at 49.45 mbsf (Fig. F6A). After an ~6 mM offset to lower concentrations between 49.45 and 54.45 mbsf, concentrations are between 14 and 19 mM to the bottom of Hole 1227A. The range in alkalinity at this site is ~21 mM. DIC exhibits a similar profile, with values increasing from 3.2 mM near the sediment/water interface to a maximum of 23 mM at 42 mbsf (Fig. F6B). The DIC concentration decreases to ~18 mM at the bottom of Hole 1227A with a similar offset between 49.45 and 54.45 mbsf. The range in DIC concentration is ~26 mM.
Maximum alkalinity and DIC concentrations at Site 1227 are considerably higher than those at open-ocean Sites 1225 and 1226. These higher values strongly suggest that rates of microbial activity are higher at Site 1227 than at the open-ocean sites. TOC content is generally high, with values ranging from 1% to >10% (Table T7; Fig. F6A, both in the "Site 1230" chapter). These high concentrations of organic carbon largely derived from algal debris (hydrogen index values of up to 500 mg HC/g TOC (Table T7 in the "Site 1230"
chapter) support the high microbial activity at Site 1227.
Sulfate penetrates to 42 mbsf, below which the concentrations remain at or near zero (Fig. F6C). Nonzero values at depths below 42 mbsf are assumed to be due to slight seawater contamination during drilling or sulfide oxidation during sampling. The sulfate profile over the uppermost 42 mbsf is complex. Sulfate concentrations drop steeply from the seawater value of 28.9 mM near the sediment/water interface to ~6 mM at 15 mbsf, probably indicating high rates of sulfate reduction in the upper sediment column. Between 15 and 42 mbsf, the gradient is less steep and includes an ~1-mM offset at ~35 mbsf. Sulfate overlaps with upward-migrating methane from 36 to 42 mbsf. Most anaerobic oxidation of methane takes place in this interval at this site. There is similar overlap between sulfate and upward-diffusing dissolved barium over the same depth interval.
Interstitial water barium concentrations (Fig. F6D) change in accordance with the sulfate distribution and may be controlled by barite solubility. Barium is close to the detection limit (~0.1 然) near the sediment/water interface and slowly rises to 2 然 at 35 mbsf. Below this depth, which coincides with a major increase in headspace methane, dissolved barium concentrations increase by over two orders of magnitude to nearly 346 然 at 145 mbsf. This rise is not steady but includes a prominent peak at 43 mbsf. The barium profile provides evidence for a zone of barite dissolution in the upper portion of the sulfate-methane transition and a zone of barite precipitation immediately below the sulfate/methane interface. White veinlets and layers, confirmed as barite by XRD, were found at 33.5 mbsf (see "Mineralogy" in "Lithostratigraphy"). The dissolved barium profile around the sulfate/methane interface may indicate that the upward migration methane has increased in the recent past (Dickens, 2001).
In contrast to the deep-sea Sites 1225 and 1226, the dissolved manganese profile at Site 1227 (Fig. F6E) shows considerable scatter and low concentrations (0-7 然) over the upper 60 mbsf. Below 66 mbsf, dissolved manganese concentrations slowly rise to 2 然 at 145 mbsf. Site 1227 is located at the lower boundary of a strong oxygen minimum zone in the overlying water column. Solid manganese probably becomes reduced and dissolves in the upper few centimeters of the sediment column or water column, which minimizes burial of solid manganese in the sediment.
Dissolved iron concentrations (Fig. F6F) remain near the detection limit (~0.2 然) down to ~100 mbsf with two exceptions. The upper 2 mbsf shows a 2-然 decrease, and a few samples between 45 and 65 mbsf have concentrations of 2-5 然. Below 100 mbsf, dissolved iron concentrations rise to 30 然. Dissolved iron concentrations are limited by sulfide solubility and the dissolved sulfide distribution. Most of the labile iron entering the sediment column probably precipitates as sulfides.
Dissolved strontium concentrations (Fig. F6G) rise from 90 然 at the seafloor to 1025 然 at 145 mbsf. Unlike chloride, however, the profile is not linear but has concave-upward curvature between the seafloor and ~50 mbsf and concave-downward curvature between 50 and 144 mbsf. A prominent 50-然 drop in strontium is also present at ~35 mbsf. The overall 6.45-然/m rise in strontium is one of the most extreme strontium gradients recorded in the marine realm and implies a substantial flux of strontium from deep brines along the Peru margin to shallow sediment and seawater. The curvature in the strontium profile from 0 to 25 mbsf and the marked deviation at ~35 mbsf probably reflect zones of local strontium removal to carbonate and barite, respectively.
Dissolved lithium concentrations (Fig. F6H) rise from 27 然 near the seafloor to 1435 然 near the bottom of the hole. At nearly 10 然/m, the increase in lithium is even greater than that for strontium. Its profile is less curved than that of strontium. As with strontium, there is a substantial flux of lithium from deep brines along the Peru margin to shallow sediment and seawater.
Total dissolved sulfide (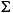 H2S = H2S + HS-) is present at a concentrations >0.043 mM in the uppermost 0.24 mbsf and increases with increasing depth to a peak concentration of ~9 mM at 38.9 mbsf (Fig. F6I). The sulfide peak coincides with the depth of the sulfate/methane interface. The shape of the sulfide curve over the 0- to 38-mbsf interval is convex upward, which suggests sulfide production via bacterial sulfate reduction throughout this interval. Below 38.9 mbsf, dissolved sulfide concentrations abruptly decrease with increasing depth. The general concave-downward shape of the sulfide profile between 40 and 75 mbsf suggests removal of sulfide, perhaps due to reactions with iron minerals (see "Magnetic Susceptibility" in "Physical Properties"). Below 75 mbsf, sulfide concentrations decrease steadily to 0 mM at 144 mbsf.
H2S = H2S + HS-) is present at a concentrations >0.043 mM in the uppermost 0.24 mbsf and increases with increasing depth to a peak concentration of ~9 mM at 38.9 mbsf (Fig. F6I). The sulfide peak coincides with the depth of the sulfate/methane interface. The shape of the sulfide curve over the 0- to 38-mbsf interval is convex upward, which suggests sulfide production via bacterial sulfate reduction throughout this interval. Below 38.9 mbsf, dissolved sulfide concentrations abruptly decrease with increasing depth. The general concave-downward shape of the sulfide profile between 40 and 75 mbsf suggests removal of sulfide, perhaps due to reactions with iron minerals (see "Magnetic Susceptibility" in "Physical Properties"). Below 75 mbsf, sulfide concentrations decrease steadily to 0 mM at 144 mbsf.
Acetate and formate were analyzed in 30 IW samples from Holes 1227A and 1227D and 12 samples from Hole 1227D to better define their concentrations in intervals where sulfate decreases (Table T2; Fig. F6J, F6K). Concentrations of both species are generally higher than those at Sites 1225 and 1226, with the exception of the lowermost 80 m of Site 1226 (see "Biogeochemistry" in the "Site 1225" chapter and "Biogeochemistry" in the "Site 1226" chapter). Minimum concentrations of acetate exist in two intervals. The first of these intervals is the upper 10 mbsf and the second is from 30 to 50 mbsf. These two intervals include the two depth zones where the sulfate profile suggests maximum rates of sulfate reduction. Apart from these two intervals, acetate concentrations rise with increasing burial depth—a general trend also noted at Site 1226. Acetate concentrations below 100 mbsf are 3.5-6 然. With regard to minimum concentrations, the profile of formate resembles that of acetate except that the overall variability in formate is higher than that of acetate (Fig. F6K). In the majority of samples, formate concentrations are <3 然. In contrast to acetate, no significant downhole increase in formate concentrations is evident.
Methane was detected in all samples at Site 1227 (Table T3; Fig. F6L). Methane concentrations increase steadily with depth and reach 55 然 in the sulfate-methane transition zone (~35-38 mbsf). In more deeply buried sediments, methane concentrations increase sharply and reach a plateau of maximum concentration below a depth of 47.9 mbsf. Below that depth, concentrations vary from ~0 to 3400 然. We suggest that these changes reflect changes in lithology and corresponding effects on methane degassing upon core retrieval rather than "real" changes in interstitial water concentrations of methane (Paull, Matsumoto, Wallace, and Dillon, 2000).
Low concentrations of ethane and propane were detected in the majority of the samples, with ethane concentrations reaching a maximum of ~3 然 (Fig. F6M). The overall depth profile of ethane approximately tracks that of methane, although the methane/ethane ratio increases from ~50 between 10 and 30 mbsf to values >103 below 50 mbsf (Fig. F6N). Notably, there are lows in ethane concentrations and the methane/ethane ratio at ~35 mbsf. This suggests that some fraction of ethane is consumed in the sulfate-methane transition zone. Propane was not detected in sediments shallower than the transition zone. Below 38.40 mbsf, propane levels approach about one-third of ethane levels (Fig. F6O).
Consistent with observations from other Leg 201 sites, the 1-day headspace extraction procedure resulted in higher gas yields compared to the 20-min extraction for safety purposes (Table F3).
Hydrogen incubations were conducted on 15 samples from Hole 1227A and 6 samples from Hole 1227D (Table T4; Fig. F6P). Hydrogen concentrations are between ~0.3 and 1.2 nM, with most samples between 0.3 and 0.5 nM. Hydrogen concentrations do not change across the sulfate-methane transition zone.
A total of 58 IW samples were analyzed for ammonium, 40 from Hole 1227A and 18 from Hole 1227D. Concentrations of ammonium were below detection in the WSTP sample and at 0.6 mbsf in Hole 1227D. They reached 680 然 at 1.35 mbsf in Hole 1227A (Fig. F6Q). In both holes, ammonium concentrations steadily increase downhole, reaching 22,500 然 at the bottom of Hole 1227A (144.45 mbsf). This is similar to the profile observed at Site 684 (Shipboard Scientific Party, 1988).
Results of phosphate analyses on 39 alkalinity samples (see "Biogeochemistry" in the "Explanatory Notes" chapter) from Holes 1227A and 1227D (Fig. F6R) render a profile that bears little resemblance to that from Site 684 (Shipboard Scientific Party, 1988). Phosphate concentrations increase sharply in Hole 1227D, from 2.3 然 at the sediment/water interface (WSTP sample) to 8.3 然 at 0.6 mbsf, and fluctuate between 2 and 10 然 from 24 to 54 mbsf. However, a broad minimum and a broad maximum are present between 24 and 40 mbsf and 41 and 54 mbsf, respectively. Phosphate concentrations appear to maintain a fairly steady value of ~5 然 from 54 to 106 mbsf. Interestingly, dissolved phosphate and magnetic susceptibility exhibit somewhat similar profiles (Fig. F7).
A total of 50 samples were analyzed for dissolved silica at Site 1227, 33 from Hole 1227A and 17 from Hole 1227D. Silica correlates well with major changes in lithology (Fig. F6S). Concentrations increase rapidly from 535 然 at the sediment/water interface to between 1000 and 1100 然 from 8.4 to 13 mbsf. A dissolved silica minimum between 14 and 22 mbsf and a pronounced maximum between 40 and 55 mbsf coincide with variations in lithology from diatom-bearing ooze to the more terrigenous glauconite- and pyrite-bearing silty layers of lithostratigraphic Unit III (see "Lithostratigraphy"). Thus, and in contrast to the open-ocean sites, dissolved silica concentrations are apparently lower in the diatom-bearing units. The relatively stable silica concentrations of 900 to 1000 然 maintained over the lower 90 m of the hole (lithostratigraphic Unit IV) likely reflects solubility control of IW silica by opal phases as the sediments return to predominantly diatom-rich oozes and clays.
Chloride concentrations were determined by ion chromatography. Chloride concentrations increase monotonically from 555 mM near the sediment/water interface to a maximum of 1178 mM at a depth of 164 mbsf (Fig. F6T). This trend is due to the upward diffusion of chloride from brine deeper in the sediment column. There is a slight convex-upward curvature to the profile. This curvature may result from upward advection or from decreasing diffusivity with depth. If it is assumed that diffusivity is constant, advection velocities >0.02 cm/yr would produce greater curvature than that observed.
Lithium increases downhole nearly linearly with chloride. Lithium increases by a factor of ~60, whereas chloride increases by a factor of ~2 (Fig. F6H, F6T). This correlation provides strong evidence that the deeply buried brine is a residual solution produced during evaporite formation rather than the dissolution of evaporitic minerals, as lithium is concentrated in residual brines and excluded from halite. The extent of lithium enrichment in a brine depends on the extent of net evaporation of the original seawater. Hence, assuming no other sources or sinks for lithium, its concentration in brine can be used to calculate the extent of evaporation. As enrichment in lithium relative to chloride occurs following halite precipitation, the lithium content of the deeply buried brine can be estimated by extrapolating the lithium-chloride correlation to halite saturation. Halite saturates at ~6 M, which is equivalent to an approximately tenfold evaporative concentration of seawater. Using the measured lithium-chloride correlation and extrapolating to halite saturation, the inferred lithium concentration in the brine buried at Site 1227 is 0.014 M. Assuming that the lithium concentration in the initial fluid is similar to that in modern seawater, the inferred brine concentration corresponds to an evaporative concentration of a factor of ~500 during brine formation.

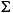 H2S = H2S + HS-) is present at a concentrations >0.043 mM in the uppermost 0.24 mbsf and increases with increasing depth to a peak concentration of ~9 mM at 38.9 mbsf (Fig. F6I). The sulfide peak coincides with the depth of the sulfate/methane interface. The shape of the sulfide curve over the 0- to 38-mbsf interval is convex upward, which suggests sulfide production via bacterial sulfate reduction throughout this interval. Below 38.9 mbsf, dissolved sulfide concentrations abruptly decrease with increasing depth. The general concave-downward shape of the sulfide profile between 40 and 75 mbsf suggests removal of sulfide, perhaps due to reactions with iron minerals (see "Magnetic Susceptibility" in "Physical Properties"). Below 75 mbsf, sulfide concentrations decrease steadily to 0 mM at 144 mbsf.
H2S = H2S + HS-) is present at a concentrations >0.043 mM in the uppermost 0.24 mbsf and increases with increasing depth to a peak concentration of ~9 mM at 38.9 mbsf (Fig. F6I). The sulfide peak coincides with the depth of the sulfate/methane interface. The shape of the sulfide curve over the 0- to 38-mbsf interval is convex upward, which suggests sulfide production via bacterial sulfate reduction throughout this interval. Below 38.9 mbsf, dissolved sulfide concentrations abruptly decrease with increasing depth. The general concave-downward shape of the sulfide profile between 40 and 75 mbsf suggests removal of sulfide, perhaps due to reactions with iron minerals (see "Magnetic Susceptibility" in "Physical Properties"). Below 75 mbsf, sulfide concentrations decrease steadily to 0 mM at 144 mbsf.