GEOCHEMISTRY
Concentrations of headspace and vacutainer gases were routinely monitored in Hole 1234A sediments according to shipboard safety and pollution prevention considerations. The high gas pressure in the cores required perforating the core liners to prevent excessive core expansion. Low methane concentrations were first detected in the shallowest headspace gas sample at 1.5 mcd (Fig. F21; Table T12). Methane (C1) increased rapidly by 9.7 mcd, and vacutainer samples had high methane concentrations (>95 vol%) at all depths. Low ethane (C2) concentrations were detected in the vacutainer samples. Ethane values gradually increase with depth, from 2.7 ppmv at 31.1 mcd to 81 ppmv at 221.3 mcd. No significant amounts of higher molecular weight hydrocarbons were observed.
High methane concentrations, low ethane concentrations, and the resulting high C1/C2 ratios (Fig. F21) indicate that the methane originates from in situ fermentation (methanogenesis) of sedimentary organic matter. A biogenic origin for methane is supported by the disappearance of dissolved sulfate by 9.7 mcd, where methane increased, because interstitial sulfate inhibits methanogenesis in marine sediments (Claypool and Kvenvolden, 1983).
Interstitial Water Geochemistry
We collected 22 interstitial water samples from Hole 1234A. Chemical gradients at this site (Table T13; Fig. F22) reflect the influence of organic matter diagenesis by microbially mediated oxidation reactions, a limited degree of biogenic opal dissolution, and the effects of authigenic mineralization reactions on fluid composition.
Chlorinity increases from 550 mM at 1.5 mcd to 572 mM at 51.8 mcd then decreases to values <565 mM by 87.9 mcd (Fig. F22). Salinity, measured refractively as total dissolved solids, increases from 35 to 37 from 29.7 to 51.8 mcd then decreases to values typically 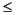 33 by 111.5 mcd (Table T13). Sodium concentrations measured by inductively coupled plasma-atomic emission spectrophotometry averaged 3% lower than those estimated by charge balance reported here (Table T13). Sodium concentrations parallel salinity and chlorinity, with a total range from 470 mM at 1.5 mcd to a maximum of 504 mM at 51.8 mcd.
33 by 111.5 mcd (Table T13). Sodium concentrations measured by inductively coupled plasma-atomic emission spectrophotometry averaged 3% lower than those estimated by charge balance reported here (Table T13). Sodium concentrations parallel salinity and chlorinity, with a total range from 470 mM at 1.5 mcd to a maximum of 504 mM at 51.8 mcd.
Organic matter diagenesis, driven by microbial oxidation reactions, dominates many of the interstitial water profiles. The relatively low organic carbon contents are apparently counterbalanced by high total sedimentation rates to result in pronounced depth variations in interstitial water chemistry, as typically observed in more organic carbon-rich, continental margin settings. Sulfate concentration at 1.5 mcd is 18.2 mM, already substantially lower than typical seawater values of 29 mM, and concentrations from 9.7 to 111.5 mcd are below the detection limit (~0.6 mM). Sulfate increases slightly with greater depth to values as high as 4 mM at 189.6 mcd and concentrations of 2-6 mM to the base of the drilled section. This minor sulfate maximum is coincident with a zone of locally elevated alkalinity, although it may reflect drilling fluid contamination.
Organic matter decomposition by sulfate reduction and methanogenesis drives large increases in alkalinity, which increases to peak values of >60 mM from 17.9 to 51.8 mcd then decreases to values <20 mM from 132.8 to 166.8 mcd. A secondary alkalinity maximum, with values >20 mM, occurs from 175.0 to 210.8 mcd. Lower alkalinity values correspond to the depth interval with a higher proportion of authigenic calcite (>120 mcd) (see "Lithostratigraphy"), indicating that authigenic mineralization reactions of inorganic carbonates are an effective alkalinity sink in these sediments.
The profiles of the reduced forms of the secondary oxidants manganese and iron show variations with depth, although not simply related to that of sulfate. Dissolved manganese concentrations average 1 然, with a small maximum at 9.7 mcd (2.5 然) and an isolated peak value (2.1 然) at 189.6 mcd. Dissolved iron concentrations have a broad maximum of >10 然 from 29.7 to 51.8 mcd, decrease to minimum values of <4 然 from 74.7 to 144.1 mcd, increase to 7.6 然 at 166.8 mcd, and then decrease with increasing depth.
Organic matter decomposition generates increases in phosphate and ammonium in interstitial water. Phosphate concentrations are >120 然 from 9.7 to 51.8 mcd and are <100 然 from 99.3 to 154.1 mcd, with a secondary maximum from 167.8 to 199.1 mcd approximately centered on the minor sulfate peak. Ammonium concentrations increase from 1.2 mM at 1.5 mcd to values >11 mM from 40.4 to 87.9 mcd and remain >8 mM throughout. Ammonium concentrations at Site 1234 are significantly higher throughout than even the peak values at Site 1233, indicating that the balance of ammonium generation by organic matter degradation and ammonium consumption differs for the two sites. The maximum in ammonium concentration is broader, deeper, and more smoothly defined than the phosphate maxima.
Dissolved silicate concentrations average ~720 然 with little depth variation (Fig. F22). Mean values are ~25% higher than those at Site 1233 but are undersaturated with respect to biogenic opal. Undersaturation may reflect the limited amount of biogenic opal available for dissolution or other controls on opal solubility, such as effects of opal composition, in these sediments. Barium concentrations are generally higher than those at Site 1233 but lack the sharp subsurface maximum seen there. Both sites experience complete depletion of seawater sulfate at shallow depths, which would drive barite dissolution and increase interstitial water barium concentrations. Barium concentrations are generally elevated in the zone of maximum alkalinity (e.g., 25 然 at 74.7 mcd) but decrease to minimum values from 99.3 to 111.5 mcd. Boron concentrations increase to values >840 然 from 17.9 to 62.9 mcd then generally decrease with increasing depth to 443 然 at 235.1 mcd. The resemblance of the boron and ammonium profiles indicates that adsorption/desorption reactions may influence the boron profile, although the maximum boron values are found shallower than maximum ammonium concentrations.
Calcium concentrations decrease sharply from 7.5 mM at 1.5 mcd to values generally <2 mM from 9.7 to 111.5 mcd then increase to >2.8 mM by 189.6 mcd. Magnesium concentrations increase to >58 mM from 17.9 to 51.8 mcd then generally decrease with increasing depth to 38 mM at 235.1 mcd. A broad minimum, with values <40 mM, occurs from 122.8 to 166.9 mcd, starting in the zone of increased authigenic carbonate (see "Lithostratigraphy"). The very strong decrease in calcium by 9.7 mcd is consistent with authigenic mineralization reactions driven by the alkalinity increase. Increasing magnesium/calcium ratios with the calcium decrease indicate that calcite precipitation is the most likely reaction taking place in shallow sediments. Magnesium/calcium ratios are generally >30 from 9.7 to 87.9 mcd but decrease to ~12 in the deepest samples from 223.7 to 235.1 mcd.
Lithium concentrations first decrease sharply then generally increase with increasing depth before decreasing in the deepest samples. Strontium concentrations decrease with increasing depth to a broad minimum before increasing to 67 然 at 235.1 mcd, and potassium concentrations increase to >13 mM from 17.9 to 74.7 mcd, then decrease with increasing depth before increasing again in the deepest three samples (Fig. F22).
Sedimentary Inorganic Carbon and Organic Carbon, Nitrogen, and Sulfur Concentrations
Inorganic carbon (IC), TOC, total nitrogen (TN), and total sulfur (TS) concentrations were determined on sediment samples from Hole 1234A (Table T14). Organic matter carbon/nitrogen ratios and Rock-Eval pyrolysis were employed to characterize the organic matter.
Calcium carbonate concentrations range between 0.8 and 11.7 wt% (average = 3.5 wt%) (Fig. F23). In the uppermost 115.8 mcd, calcium carbonate concentrations vary around a mean of 3.0 wt%. A pronounced carbonate maximum up to 11.7 wt% is present between 118.6 and 123.6 mcd. At greater depths, the carbonate contents decrease gradually from ~6.0 to ~3.0 wt%. Calcium carbonate originates mainly from calcareous plankton, although diagenetically formed IC is visible in some intervals (see "Lithostratigraphy"). Low calcium carbonate concentrations in sediment from throughout the hole most likely reflect dilution by terrigenous sediment components. Changes in the carbonate contents may indicate changes in the fluvial supply of siliciclastics (see "Lithostratigraphy"), although fluctuations in carbonate flux may also be linked to changes in surface productivity triggered by the upwelling zone of Concepci鏮 (Chile). Fluctuations in carbonate dissolution may also have contributed to the variations in carbonate content because preservation of calcareous microfossils varied from poor to good (Tables T9, T10).
TOC concentrations range between 0.2 and 3.0 wt% (average = 1.1 wt%) (Fig. F23). The shallowest sample at 0.7 mcd has a high value of 2.0 wt%. TOC concentrations remain around a mean value of 1.0 wt% to a depth of 50 mcd, with small fluctuations. At greater depths, a more TOC-rich interval is observed between 54.5 and 90.2 mcd, with concentrations typically >1.5 wt% and up to 3.0 wt%. At greater depth, TOC concentrations are similar to the uppermost 50 mcd, but with larger fluctuations. Another maximum in TOC of 2.3 wt% is present at 223 mcd. The TN record contains very similar trends (Fig. F23). The good correlation between TOC concentrations and diatom abundance (see "Biostratigraphy") suggests that the variations observed in both indicators should be linked primarily to productivity changes.
These upper Pleistocene sediments are poor in TOC compared to other coastal upwelling systems. For instance, in the Namibia and California margins, upper Pleistocene TOC concentrations are as high as 17 and 7 wt%, respectively (Lyle, Koizumi, Richter, et al., 1997; Berger et al., 1998). Lower TOC concentrations in the Chile margin sediments most likely result from greater dilution with siliciclastic material.
TS concentrations are high throughout the record, varying between 0.2 and 2.1 wt% (Table T14). In the uppermost 120 mcd, the TS profile presents large fluctuations (Fig. F23). TS concentrations gradually increase below 120 mcd to reach a maximum between 210 and 220 mcd then decrease. This TS maximum, which corresponds to the maximum in TOC and TN concentrations, could be attributed to the formation of pyrite during the microbial degradation of the organic matter by sulfate reduction.
TOC/TN ratios typically range between 5 and 10, which indicates a predominantly marine origin of the organic material (Fig. F24) (Bordovskiy, 1965; Emerson and Hedges, 1988; Meyers, 1997). Lower TOC/TN ratios are associated with lower TOC concentrations (Fig. F24), indicating that terrigenous organic matter supply and/or preservation effects during diagenesis control at least part of the organic matter variations.
Seven samples with different TOC concentrations and TOC/TN ratios were selected for Rock-Eval measurements. Low Tmax values indicate that the organic matter is thermally immature (Table T15). The relationship between S2 and TOC shows that the organic matter is dominantly Type II (Fig. F25) (i.e., marine algal organic matter) (Tissot and Welte, 1984; Langford and Blanc-Valleron, 1990), which is consistent with the TOC/TN ratios.
Fresh marine plankton has a relatively high lipid content, thus high H/C ratios. Therefore, well-preserved organic matter of marine algal origin yields high hydrogen index (HI) values when subjected to pyrolysis. The HIs measured in these samples range from 189 to 299 (Table T15), which indicates that the organic matter in sediments from the Chile margin is significantly degraded. Moreover, keeping in mind that TOC contents are low throughout the sedimentary record and that only a few samples have been measured with the Rock-Eval, the correspondence between decreases in both TOC concentrations and HI values (Fig. F25) could indicate that preservation of marine organic matter during diagenesis is important in controlling the organic carbon contents of sediments on the Chile margin.

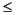 33 by 111.5 mcd (Table T13). Sodium concentrations measured by inductively coupled plasma-atomic emission spectrophotometry averaged 3% lower than those estimated by charge balance reported here (Table T13). Sodium concentrations parallel salinity and chlorinity, with a total range from 470 mM at 1.5 mcd to a maximum of 504 mM at 51.8 mcd.
33 by 111.5 mcd (Table T13). Sodium concentrations measured by inductively coupled plasma-atomic emission spectrophotometry averaged 3% lower than those estimated by charge balance reported here (Table T13). Sodium concentrations parallel salinity and chlorinity, with a total range from 470 mM at 1.5 mcd to a maximum of 504 mM at 51.8 mcd.