INTERPRETATION AND APPLICATION
The fundamental principle of maturity evaluation by biomarker analysis is based on the structural modification of compounds by stepwise transformation from biological precursor molecules to thermodynamically more stable geochemical fossils. Generally, the form of biologic precursor molecules is defined by their function (e.g., as a part of the cell membrane) and not by thermodynamic stability of the structure. Diagenetic pathways lead to sequential structural rearrangements to more stable molecules, lowering enthalpy while increasing entropy. Thermodynamic equilibrium is eventually reached, which defines the endpoint of the transformation and determines the final ratio of isomers or products.
The biomarker composition of the apolar fractions of all samples is dominated by hopanes and steranes retaining their biological, and therefore thermally immature, structural configurations. Selected maturity parameters based on biomarkers (Table T2) show no major differences between the two sites in terms of overall maturity. A low thermal maturity is indicated by the presence of 5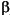

 -R C27 to C29 steranes along with the more abundant 5
-R C27 to C29 steranes along with the more abundant 5

 -R isomers. 20S-isomers of the latter compounds were not detected, nor were their 5
-R isomers. 20S-isomers of the latter compounds were not detected, nor were their 5
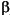
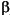 -configurations.
-configurations. 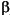
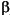 -hopanes dominate over all other homologous series of hopane isomers, and the abundance of 22S-isomers is significantly lower than that of 22R-isomers for C30 and C31 (Table T2), consistent with sediment immaturity.
-hopanes dominate over all other homologous series of hopane isomers, and the abundance of 22S-isomers is significantly lower than that of 22R-isomers for C30 and C31 (Table T2), consistent with sediment immaturity.
For most samples, a significant suite of different sterenes and hopenes is present, indicating low thermal maturity of the organic matter, which agrees with the low Tmax values of the Rock-Eval pyrolysis (Table T1). In addition, it suggests little thermal modification of the kerogen, which implies that the n-alkane distribution of the samples likely represents their unaltered, original composition. Measures, such as the CPI, that are affected by maturation can therefore be applied, although low maturity also produces a potential bias in the distributions of free biomarker fractions because they fail to reflect contributions of organic matter still bound into the kerogen or other macromolecular fractions (Koopmans et al., 1996a). The homohopane index may reflect this bias because the abundance of compounds formed from pentakishomohopanes might be expected to be limited until catagenesis (K÷ster et al., 1997). Similarly, the pristane/phytane ratio may be influenced by maturity because the abundance of both compounds in the free hydrocarbon fraction is strongly affected by thermal generation of these compounds from precursors and liberation from the bound biomarker fraction (Koopmans et al., 1999). Consequently, the pristane/phytane ratio as given in Table T3 should not be used for the assessment of oxygen levels in the paleoenvironment (Volkman and Maxwell, 1986, see below).
The fundamental principle in assessing of biological sources of organic matter using biomarkers is utilization of source-specific compounds. Some compounds indicate a general source (e.g., Brassell et al., 1978; Ourisson et al., 1987); others such as certain isoprenoids or carotenoids (e.g., isorenieratene) are highly source specific (e.g., Brassell et al., 1981; Volkman, 1988).
One example of the general source indicators is the short- vs. long-chain n-alkanes derived from aquatic algal/microbial and higher plant sources, respectively. For most samples, the n-alkane distribution is dominated by n-C16 to n-C18 (Table T3), indicating a dominant marine source for the n-alkanes. An odd over even carbon number predominance (given as CPI in Table T3) is observed starting at n-C23 or n-C25, with n-C29 to n-C31 being the most abundant higher range compound. The ratio of n-C17/n-C31 given in Table T3 is a simplified parameter that reflects relative contributions of aquatic/marine and terrestrial n-alkanes. Evidently, the proportion of land-plant material present in the samples varies through time. Samples 207-1258B-44R-4, 75–76 cm, and 51R-2, 40–41 cm, represent the marine end-members (Table T3), and Samples 207-1258C-31R-2, 26–27 cm, and 207-1257B-22R-1, 44–45 cm (Fig. F1B; Table T3), represent the terrestrial end-members of this binary system. Degradation of organic matter can alter the n-alkane distribution and thereby modify the source signal by enriching the more stable land-derived fraction through enhanced loss of the more labile aquatic lipids (Sinninghe DamstÚ et al., 2002). Thus, the predominant marine signature in the n-alkane distributions of most samples also suggests overall good preservational conditions.
The isoprenoids pristane and phytane are primarily derived from the phytol sidechain of the chlorophyll molecule (e.g., Didyk et al., 1978; review in Volkman and Maxwell, 1986) and can be used as biomarkers for phytoplankton. However, other primary sources, such as archaebacterial ether lipids for both molecules (Didyk et al., 1978), or tocopherols for pristane (Brassell et al., 1983a; Goossens et al., 1984), have been proposed (review in Koopmans et al., 1999). These alternate origins could complicate the source pristane-phytane interpretation in the paleoenvironmental setting at the Demerara Rise, especially possible archaeal contributions.
The source for lycopane in the marine environment is not clear, as neither the molecule itself nor potential precursors have been found in the lipids of a specific group of marine organisms yet. Possible sources are prokaryotes (methanogenic archaea) (e.g., Brassell et al., 1981) or, alternatively, photoautotrophic algae (e.g., Wakeham et al., 1993; overview in Sinninghe DamstÚ et al., 2003). Corroborating evidence for the latter source in aquatic settings stems from the stable carbon isotopic composition of lycopane analyzed in seawater and various recent and ancient marine sediments (Wakeham et al., 1993; Sinninghe DamstÚ et al., 2003) and the detection of lycopadiene in a specific strain of the green algae Bottryococcus braunii (Metzger et al., 1990). However, B. braunii is a freshwater species, and it remains unclear how lycopane is preferably generated from this unsaturated precursor (Wakeham et al., 1993). According to Sinninghe DamstÚ et al. (2003), the high abundance of lycopane in the samples investigated here more likely has stronger implications for the state of water-column oxygenation than for the rate of primary production. Comet et al. (1981) reported a lycopane-dominated n-alkane distribution with an entirely marine signature from a laminated upper Albian limestone sample (TOC = 2.1 wt%) from the Hess Rise (northwest Pacific, DSDP Leg 62, Site 465). They proposed an oxygen-depleted water-column setting analog to an oxygen-minimum zone overlain by waters characterized by high algal productivity. Examples of European and Tunisian marine Cenomanian/Turonian black shales yielding lycopane are given in Farrimond et al. (1990).
A widely used tool for paleoenvironmental and source assessment of sedimentary organic matter employs the relative abundances of the C27, C28, and C29 (

 -R steranes) (e.g., Moldowan et al., 1985; Peters and Moldowan, 1993) as proxies for their eukaryotic sterol precursors (e.g., Mackenzie et al., 1982). Originally, this approach was based on the observation that C28 sterols dominate in phytoplankton (green algae and diatoms), C27 sterols are typical for zooplankton and red algae, and C29 sterols occur in higher plants and some strains of brown or green algae (Huang and Meinschein, 1979). However, care has to be taken when utilizing sterane compositions, as these source assignments are simplified (review in Volkman, 1986, 1998). It is, for example, problematic that the biological sources of the C27 and C29 steranes that are the most dominant compounds in the Demerara Rise setting cannot actually be differentiated. There is little correlation between the abundances of the C29 sterane and n-C31 (Table T3), which implies either different terrigenous sources for both compounds or that the C29 sterane cannot be interpreted as a terrestrial marker here. Nevertheless, there are pronounced differences in the distributions of regular steranes at different stratigraphic levels (Table T3), but it remains unknown if these reflect actual changes in the composition of the algal community. As an example, the ratio of C28/C27-
-R steranes) (e.g., Moldowan et al., 1985; Peters and Moldowan, 1993) as proxies for their eukaryotic sterol precursors (e.g., Mackenzie et al., 1982). Originally, this approach was based on the observation that C28 sterols dominate in phytoplankton (green algae and diatoms), C27 sterols are typical for zooplankton and red algae, and C29 sterols occur in higher plants and some strains of brown or green algae (Huang and Meinschein, 1979). However, care has to be taken when utilizing sterane compositions, as these source assignments are simplified (review in Volkman, 1986, 1998). It is, for example, problematic that the biological sources of the C27 and C29 steranes that are the most dominant compounds in the Demerara Rise setting cannot actually be differentiated. There is little correlation between the abundances of the C29 sterane and n-C31 (Table T3), which implies either different terrigenous sources for both compounds or that the C29 sterane cannot be interpreted as a terrestrial marker here. Nevertheless, there are pronounced differences in the distributions of regular steranes at different stratigraphic levels (Table T3), but it remains unknown if these reflect actual changes in the composition of the algal community. As an example, the ratio of C28/C27-

 -R steranes more or less increases upsection at both sites (Table T3).
-R steranes more or less increases upsection at both sites (Table T3).
Hopanes represent a biomarker group derived exclusively from prokaryotic sources (Ourisson et al., 1979). Generally, they are nonspecific for any group of bacteria or other microbes. To estimate the temporal variation of contributions of eukaryotic vs. prokaryotic sources, the sum of all sterane compounds (m/z 217) and all hopanes (m/z 191) has been utilized as the sterane/hopane ratio given in Table T3. However, by employing m/z 217 for integration of the sterane compounds, this ratio appears to be biased toward enhancing the hopane group for these immature samples. The abundance of steranes/sterenes and hopanes/hopenes in the TIC biomarker spectrum as shown in Figure F2 clearly indicates that both algal and microbial sources more or less equally contribute to biomarker assemblage in high amounts. To compensate for this bias, the ratio needs to be corrected by some factor or the TIC should be used for its calculation. Nevertheless, the large proportion of prokaryotic biomarkers seems quite unusual for a normal marine setting but is well known from other marine Cretaceous black shale deposits. Examples are lower Aptian deposits (OAE 1a; Leg 198, Sites 1207 and 1213, Shatsky Rise, northeast Pacific; Bralower, Premoli Silva, Malone, et al., 2002), Turonian sediments (Leg 75, DSDP Site 530, Angola Basin, southeast Atlantic; Brassell, 1984), and Cenomanian/Turonian sediments in marine settings in Europe and Tunisia (OAE 2; Farrimond et al., 1990).
Something that remains for further investigation is the origin and significance of the high abundance of perylene in some samples (see "Aromatic
Fraction" in "Biomarkers"). The source for perylene is still not known, but it may represent an early stage diagenetic product formed in sediment from nonspecific precursors (Wakeham et al., 1979; Silliman et al., 2000).
Assessment of Paleoenvironment/Preservational Conditions
The quality of organic matter preservation can be estimated by the degree of degradation of sensitive geochemical compounds and the presence of labile compounds such as carbohydrates or pigments. As the organic matter at Sites 1257 and 1258 is immature and prokaryotic sources for phytane cannot be ruled out (see "Maturity Evaluation" and "Source Evaluation"), a classical parameter, the pristane/phytane ratio (Didyk et al., 1978) (Table T3), should not be employed as a strict measure for the degree of oxygen depletion in the water column. However, the low values stated in Table T3 are like those reported from other marine Cretaceous black shales of comparably low thermal maturity (e.g., Bralower, Premoli Silva, Malone, et al., 2002; Brassell et al., 1983b; Comet et al., 1981; Didyk et al., 1978; Meyers et al., 1984). This similarity could indeed imply that the paleoenvironmental conditions at the Demerara Rise provided a suitably reducing setting in which the generation of phytane from phytol could have taken place. However, even if we assume that thermal maturity does not have a biasing influence on the proportions of pristane and phytane here, additional prokaryotic sources for phytane cannot be ruled out.
The homohopane index (Table T2; note modification of index used here) is the percentage of the pentakishomohopanes (C35) vs. the total of all homohopanes ( C31–C35). The index is based on the premise that the longer sidechain molecules C33 to C35 are only preserved under anoxic conditions (Peters and Moldowan, 1991), for example by sulfur incorporation (K÷ster et al., 1997). In dysoxic to oxic settings, oxidation and subsequent decarboxylation of the original C35-precursor molecules lead to a shortening of the sidechain so that C31- to C32-homohopanes are generated. The samples from Site 1258 show a slightly higher homohopane index on average than those from Site 1257. If the homohopane index is not affected by other factors, this would speak to a somewhat more oxygen-depleted setting at Site 1258.
C31–C35). The index is based on the premise that the longer sidechain molecules C33 to C35 are only preserved under anoxic conditions (Peters and Moldowan, 1991), for example by sulfur incorporation (K÷ster et al., 1997). In dysoxic to oxic settings, oxidation and subsequent decarboxylation of the original C35-precursor molecules lead to a shortening of the sidechain so that C31- to C32-homohopanes are generated. The samples from Site 1258 show a slightly higher homohopane index on average than those from Site 1257. If the homohopane index is not affected by other factors, this would speak to a somewhat more oxygen-depleted setting at Site 1258.
Hopanoid thiophenes are present in all samples, which suggests that sulfur incorporation into the organic matter has taken place throughout the entire black shale sequence at both sites, most likely at an early diagenetic stage (e.g., Werne et al., 2000). The ratio of hopanoid thiophenes over C30-hopanes is also slightly higher in samples from Site 1258 than in those from Site 1257 (Table T2). This might point toward a higher rate of sulfur incorporation into the organic matter at Site 1258 if influences by other factors (e.g., differences in the abundance of precursor molecules or sedimentation rates) can be ruled out. Incorporation of inorganic sulfur species into organic matter enhances the preservation of labile compounds such as carotenoids or carbohydrates (e.g., Kok et al., 2000) but also implies that a substantial quantity of these reactive compounds is captured in the bound-biomarker fraction of these immature samples. In this case, paleoenvironmental interpretations cannot be based solely on the abundance of isorenieratane or its derivatives in the free-biomarker fraction but need to be corroborated by analysis of the sulfur-bound isorenieratane.
Kuypers et al. (2002) found sulfur-bound isorenieratane in elevated concentrations in sediments from just below the Cenomanian/Turonian boundary at DSDP Site 144, which has been redrilled as Site 1257 during Leg 207. They also reported its occurrence in lower concentrations for sediments below the onset of the isotopic excursion of OAE 2 and concluded that sulfur-containing anoxic water masses must have periodically reached the photic zone before the onset of OAE 2. Our investigation at Sites 1257 and 1258 was limited to the free-biomarker fraction and mainly isorenieratane thianes that were found in five samples (Table T2); hence, further interpretations should be reserved until more comprehensive studies, including the bound-biomarker fraction, are done. Interestingly, isorenieratane and several other isorenieratane derivatives were found in the aromatic fraction of Sample 207-1258B-46R-2, 56–57 cm. This sample is unusually rich in organic matter (TOC = 28 wt%) (Table T2) and, according to biostratigraphy, is situated closely below the Cenomanian/Turonian boundary (see
"Biostratigraphy" in the "Site 1258" chapter), which probably places it within the OAE 2 range.
Lycopane offers perhaps the best potential for addressing questions of oxygen paleolevels in the water column, as its concentration in the free hydrocarbon biomarker fraction is not a function of thermal maturity and it is not known to be prone to diagenetic sulfurization (Sinninghe DamstÚ et al., 2003). Sinninghe DamstÚ et al. (2003) propose the ratio of lycopane vs. n-C31 as a measure for the paleoredox conditions during sediment deposition based on data from the oxygen minimum zone (OMZ) of today's Arabian Sea. They reported an increase of the (lycopane + n-C35)/n-C31 ratio from values of ~0.3 for sediments above and below the OMZ to unity or even higher within the OMZ. The ratios for (lycopane + n-C35)/n-C31 given in Table T2 are >1 for all but the Albian sample. However, the amount of terrestrially derived long-chain n-alkanes (namely n-C31) varies significantly in these samples, which affects the ratio, yet the high abundance of lycopane implies that black shale sedimentation took place under strongly oxygen depleted conditions. Better constrains on the sedimentation rates estimated for the black shale interval (0.5 cm/k.y. for Site 1257 and 0.3 cm/k.y. for Site 1258) (see "Sedimentation
Rates" in the "Site 1257" chapter and "Sedimentation
Rates" in the "Site 1258" chapter) would help improve these paleoenvironmental interpretations.

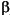

 -R C27 to C29 steranes along with the more abundant 5
-R C27 to C29 steranes along with the more abundant 5

 -R isomers. 20S-isomers of the latter compounds were not detected, nor were their 5
-R isomers. 20S-isomers of the latter compounds were not detected, nor were their 5
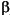
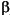 -configurations.
-configurations. 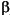
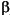 -hopanes dominate over all other homologous series of hopane isomers, and the abundance of 22S-isomers is significantly lower than that of 22R-isomers for C30 and C31 (Table T2), consistent with sediment immaturity.
-hopanes dominate over all other homologous series of hopane isomers, and the abundance of 22S-isomers is significantly lower than that of 22R-isomers for C30 and C31 (Table T2), consistent with sediment immaturity.
 C31–C35). The index is based on the premise that the longer sidechain molecules C33 to C35 are only preserved under anoxic conditions (Peters and Moldowan, 1991), for example by sulfur incorporation (K÷ster et al., 1997). In dysoxic to oxic settings, oxidation and subsequent decarboxylation of the original C35-precursor molecules lead to a shortening of the sidechain so that C31- to C32-homohopanes are generated. The samples from Site 1258 show a slightly higher homohopane index on average than those from Site 1257. If the homohopane index is not affected by other factors, this would speak to a somewhat more oxygen-depleted setting at Site 1258.
C31–C35). The index is based on the premise that the longer sidechain molecules C33 to C35 are only preserved under anoxic conditions (Peters and Moldowan, 1991), for example by sulfur incorporation (K÷ster et al., 1997). In dysoxic to oxic settings, oxidation and subsequent decarboxylation of the original C35-precursor molecules lead to a shortening of the sidechain so that C31- to C32-homohopanes are generated. The samples from Site 1258 show a slightly higher homohopane index on average than those from Site 1257. If the homohopane index is not affected by other factors, this would speak to a somewhat more oxygen-depleted setting at Site 1258.