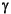 = ~90°), most of the plagioclase was previously classified as bytownite (Stephen, Kasahara, Acton, et al., 2003), but EMP analyses indicate an andesine-labradorite composition, with anorthite content ranging from An36 to An71 (Fig. F2).
= ~90°), most of the plagioclase was previously classified as bytownite (Stephen, Kasahara, Acton, et al., 2003), but EMP analyses indicate an andesine-labradorite composition, with anorthite content ranging from An36 to An71 (Fig. F2).
We report compositions of glasses, adjacent spherulitic portions of pillow rims, principal silicate minerals, Fe-Ti oxides, and some alteration products for 13 basalt samples from Site 1224, for a total of >120 analyses. For these analyses, we utilized a Cameca SX 50 electron microprobe at the Istituto di Geologia Ambientale e Geoingegneria of the Dipartimento di Scienze della Terra of the University of Rome La Sapienza (Italy). Setting conditions are 15 kV and 15nA, whereas the electron beam diameter ranges from 5 µm for oxides, pyroxene, olivine, and plagioclase to 20 µm for glass and myrmekitic intergrowth.
Plagioclase is the most common groundmass and phenocryst phase. The chemical analyses of plagioclase, An-Ab-Or content, and atomic formulas calculated on the basis of 32 oxygens are reported in Table T1. On the basis of optical properties (e.g., 2V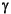 = ~90°), most of the plagioclase was previously classified as bytownite (Stephen, Kasahara, Acton, et al., 2003), but EMP analyses indicate an andesine-labradorite composition, with anorthite content ranging from An36 to An71 (Fig. F2).
= ~90°), most of the plagioclase was previously classified as bytownite (Stephen, Kasahara, Acton, et al., 2003), but EMP analyses indicate an andesine-labradorite composition, with anorthite content ranging from An36 to An71 (Fig. F2).
Lithostratigraphic Unit 1 groundmass plagioclase composition is slightly less calcic (An50–60) than the rare phenocrysts (An63–67). Such a difference is not observed in lithostratigraphic Unit 2 plagioclase, characterized by phenocrysts and groundmass phases with overlapping An composition higher than Unit 1 plagioclase (An60–71). On the other hand, lithostratigraphic Unit 3 plagioclase shows An composition overlapping with Unit 1 groundmass plagioclase (An50–61). With the exception of very few samples, the phenocrysts are unzoned. The few zoned plagioclases show normal zoning with labradoritic core (An64–66) and andesine rim (An37–45). Groundmass plagioclase of Units 1 and 3 shows andesine composition (An36–42).
Clinopyroxene is a major groundmass phase, but it occurs only rarely as a phenocryst, in glomerocrysts, or in gabbroic clots. Chemical analyses of clinopyroxene are reported in Table T2 together with structural formulae calculated on the basis of six oxygens, the Wo-En-Fs molecular weights, Mg# [Mg# = Mg/(Mg + Fe2+)] and Cr# [Cr# = Cr/(Cr + Al)]; Fe2+ and Fe3+ proportion has been calculated using the method of Droop (1987). In the standard Ca-Mg-Fe quadrilateral diagram for pyroxenes (Morimoto et al., 1988), Site 1224 clinopyroxene is augite, albeit showing some compositional variability (Wo28–42-En36–58-Fs12–30) (Fig. F3). The Fe-rich pyroxenes are mostly confined to lithostratigraphic Unit 3; conversely, Unit 2 pyroxenes show the lowest Fe and the highest Ca (Table T2). Rare phenocrysts show very uniform chemical composition. Only one sample shows a weak normal zoning with a core richer in Ca and Mg (Wo39-En42-Fs19) and rim richer in Fe (Wo34-En43-Fs23). Phenocrysts are characterized by the lowest Fe and the highest Ca (Wo34–40-Fs12–19) of the data set (Table T2; Fig. F3).
Unaltered olivine is extremely rare. Only a single microphenocryst with Fo78 has been analyzed (Sample 200-1224F-11R-2, 13–15 cm; a pillow lava fragment of lithostratigraphic Unit 2) (Table T3). Using olivine-liquid equilibria (Roeder and Emslie, 1970) and assuming a Fe/Mg KD = 0.3, this olivine composition indicates that it crystallized in equilibrium with a moderately evolved liquid (Mg# = ~51). The monticellite molecular content (CaO = 0.35 wt%) is typical of melts equilibrated at relatively low pressures (e.g., Brey and Kohler, 1990).
Large and usually skeletal oxide minerals are ubiquitous in the groundmasses of the two flows in lithostratigraphic Unit 1, whereas oxide minerals are much smaller in pillow fragments. These minerals are both rhombohedral (ilmenite-hematite solid solutions) and octahedral (ulvöspinel-magnetite solid solutions). Minerals of the first group are much rarer than those of the second; only a couple of ilmenites were analyzed. EPM data are presented in Table T4 along with structural formulae (calculated on the basis of four oxygens for the spinels and three oxygens for the rhombohedral phases) and Mg#. Ilmenite (Ilm) and ulvöspinel (Usp) molecular content were calculated following Stormer (1983); Fe2+ and Fe3+ proportion was calculated using the stoichiometric method of Droop (1987).
The spinel phases show some compositional variability (Usp68–92), whereas the rhombohedral phases are exclusively represented by almost pure ilmenite (Ilm94–96) (Fig. F4). Titanomagnetite with the highest ulvöspinel content occurs in the lower flow of lithostratigraphic Unit 1 (Usp92), whereas spinel in the upper flow has the lowest Ti content (Usp68–73).
EMP analyses of clear volcanic glass, dark spherulitic glass, and palagonite from pillow rims and hyaloclastites are reported in Table T5; selected major elements vs. the sum of oxides are plotted in Fig. F5. The results show a strong compositional variability, mostly linked to the palagonitization stage of the alteration. The analyzed glass ranges from shards without signs of alteration to shards partially to totally altered in palagonite, belonging both to pillow fragments and hyaloclastitic breccias. Massive basalts contain volcanic glass nearly totally recrystallized to clay minerals.
The range of composition of fresh glass is SiO2 = 48.7–50.4 wt%, Al2O3 = 12.5–14 wt%, TiO2 = 2.0–2.8 wt%, FeOtot = 12.0–14.2 wt%, MgO = 5.3–7.2 wt%, CaO = 9.8–11.1 wt%, Na2O = 2.5–3.0 wt%, K2O = 0.11–0.20 wt%, and P2O5 = 0.10–0.33 wt%. The sum of oxides ranges from 97.6 to 99.4 wt% (Fig. F5).
The palagonitized glass shows a much larger range of composition (Table T5; Fig. F5) coupled with lower SiO2 (40.3–47.9 wt%) and Al2O3 (8.4–9.5 wt%), much lower MgO (1.3–3.3 wt%) and CaO (0.5–1.0 wt%), and higher TiO2 (3.5–3.8 wt%), FeOtot (19.7–23.7 wt%), and K2O (2.4–3.9 wt%). Na2O content varies, ranging from values lower than fresh glasses (0.7–1.7 wt%) to much higher values (6.5 wt%) (Table T5). Moreover, palagonitized glass shows MnO and P2O5 contents close to the limit of detection (~0.1 wt%) (Table T5). The sum of the oxides is relatively variable but is always lower than unaltered glass (84.6–92.4 wt%), a result presumed to reflect hydration during the palagonitization process.
One glassy shard (Sample 200-1224F-6R-1, 29–34 cm, Point 2-1 in Table T5) with optical features of unaltered glass has an intermediate composition. This glass shows SiO2 (49.6 wt%) and TiO2 (3.1 wt%) similar to that of unaltered glass but with lower Al2O3 (9.3 wt%) and MgO (4.8 wt%). Moreover, this glass shows FeOtot (19.7 wt%), CaO (0.7 wt%), and Na2O (0.3 wt%) within the range of the palagonitized glasses but with extremely high K2O (4.3 wt%). This sample probably represents glass at the beginning of the palagonitization stage.
Table T6 includes analyses of intergrowths of quartz and alkali feldspar found in Sample 200-1224F-3R-1, 14–16 cm, from lithostratigraphic Unit 1. To obtain an average analysis, the electron beam was defocused to a 20-µm diameter in order to prevent Na volatilization and analyze a large area. The intergrowths developed in interstitial positions between plagioclase laths in the groundmass and as rims on tabular plagioclase (Fig. F6). Shipboard petrologists interpreted this texture as the product of nearly eutectic crystallization of quartz and Na-rich plagioclase (Stephen, Kasahara, Acton, et al., 2003). EMP analyses show evidence of high Al2O3 (11.1–12.5 wt%), SiO2 (78–80 wt%), and Na2O (5.5–6.2 wt%), whereas CaO, TiO2, FeOtot, MgO, and K2O are low (generally <1 wt%) (Fig. F5).